Introduction and Overview
Physical therapists and occupational therapists are non-prescribing healthcare practitioners. You may be asking yourself, "Why do I need to know this?" If that is your mindset, I hope that we can help you to see why it's important that you become competent with basic pharmacological principles. In all 50 states, physical therapists have autonomous practice ability. Some states won't allow long-term treatment without a physician referral, but they will allow evaluation. With autonomous practice, you have a professional responsibility to screen your patients' medications and be able to point out potential issues with those medications.
Additionally, medications significantly influence your patients' ability to participate in physical therapy. When I was beginning in home health, I had been a stay-at-home mom for several years. I was working in a patient's home, and I called their physician because I was concerned that my patient had a very low heart rate. It turns out the patient was on beta blockers and I had no idea that was why their heart rate had decreased. That's one of the reasons why I decided to become a champion for physical therapists to be able to understand and implement knowledge of medication into their practice.
Medicines can significantly influence a patient's ability to participate in therapy. Conversely, therapy can significantly influence the action of certain medications. Sometimes, therapy can hasten medication metabolism. Sometimes it can slow it. Sometimes it can impact how it is metabolized. As therapists, we need to be aware of that. If we have knowledge in that area, we can optimize both the pharmacological intervention and the physical therapy intervention.
First, I will provide a brief overview of pharmacology. Next, we will discuss the most common ways in which medications are used for central nervous system disorders. We will take a look at some case examples of patient problems to apply our knowledge and discuss as a group. I will try to point out some commonalities among diagnoses and medications. Finally, I will show you how to find reliable websites that provide accurate drug information.
Pharmacology Review
Pharmacology is the study of medications. Pharmacology can be divided into two subcategories: pharmacotherapeutics and toxicology. Pharmacotherapeutics is the study of the beneficial aspects of medication. Toxicology is the study of the harmful aspects of medication, those which may even lead to death. For the purposes of today's presentation, we will solely be looking at pharmacotherapeutics, which can be divided further into two more categories: pharmacokinetics and pharmacodynamics.
Pharmacokinetics is the study of what the body does to the drug. The drug is administered, and then it is absorbed into the bloodstream. It's distributed into certain systems within the body. It is metabolized and eventually excreted. The body is trying to get rid of the drug from the moment it's administered.
Pharmacodynamics is the study of what the drug does to the body. The drug exerts an effect on the cellular level, and the combined cellular level effects cause a systemic effect that can exert an influence throughout the body.
The primary mechanism of action is the means by which a drug produces an alteration in function. That usually occurs at a cellular level, with some type of interaction with a cellular receptor. The drug binds to a cellular receptor and causes a biochemical reaction that alters the cell function. If you remember back to physiology class, we have a lipophilic bipolar cell membrane, and embedded in the membrane are glycoproteins. The glycoproteins have communication with the extracellular space and also within the cell. Often, neurotransmitters will bind to these receptors and they cause some change. The drug can also bind to the receptor to cause some action.
To reiterate and summarize pharmacotherapeutics, it is helpful to look at the following flowchart. On the upper half of Figure 1, we can see the process of pharmacokinetics: what the body does to the drug. First, a dose of a drug is administered either enterally (i.e., by mouth) or parenterally (i.e., going around the digestive system). Parenteral administration is given through an IV or via some intramuscular or subcutaneous method. After administration, the drug is absorbed and eventually makes its way into the bloodstream. After absorption, we want the medication to be distributed to particular tissues so that it can have some pharmacological effect. As this distribution is going on, the drug is also being metabolized and excreted. This administration, absorption, distribution, metabolism, and elimination is all part of pharmacokinetics.
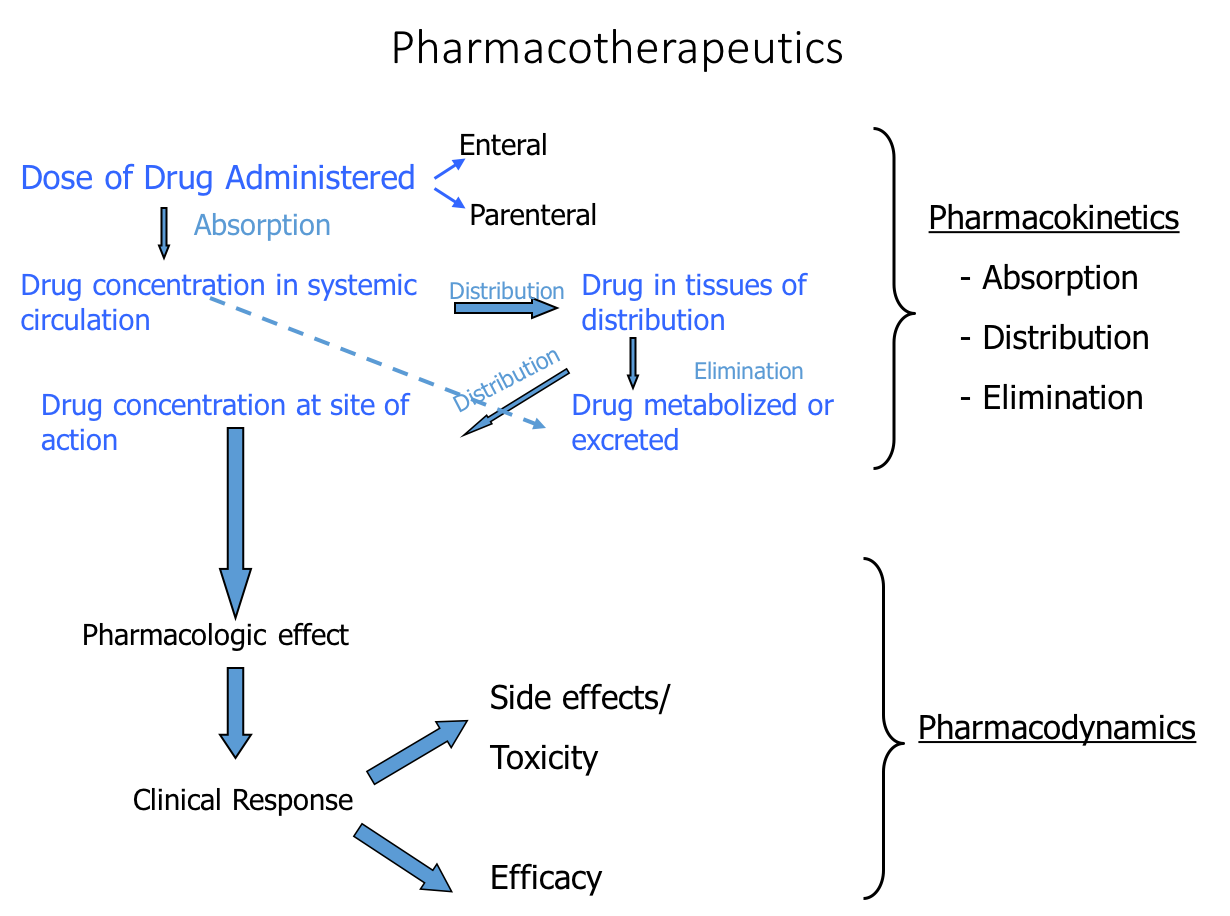
Figure 1. Processes of pharmacokinetics and pharmacodynamics.
The bottom portion of Figure 1 shows the process of pharmacodynamics: what the drug does to the body. When the drug reaches the site of action in the body in a sufficient concentration, it should have a pharmacological effect and provide some clinical response. For example, if I administered a medication for high blood pressure, the clinical response would be lowered blood pressure. Such a response as lowered blood pressure would be an efficacious response to that medication. Hopefully, our medications have a high level of efficacy and give the desired response, but medications are known to also give side effects and potentially toxic effects. That entire process is pharmacodynamics.
There are many different drugs for neurological disorders. I've tried to provide a structure for you to look at these drugs. If you encounter an unfamiliar drug, you can refer back to this structure, so you can compare and contrast the new drug with the drugs that you're familiar with.
Drugs are commonly given for central nervous system disorders for one of four purposes:
- To minimize secondary damage acutely, immediately after a traumatic event, such as:
- Spinal cord injury (SCI)
- Ischemic cerebrovascular accident (CVA)
- Traumatic brain injury (TBI)
- To manipulate neurotransmission (either to increase or decrease neurotransmission) in cases such as:
- Parkinson's disease (PD)
- Alzheimer's disease (AD)
- Psychiatric diseases
- To try to slow the disease progression, in cases such as:
- Multiple sclerosis (MS)
- Amyotrophic lateral sclerosis (ALS)
- To minimize signs/symptoms and secondary problems that may develop with neurological disorders:
- Hypertone/spasticity
Minimize Secondary Damage Following Acute Event
The central nervous system is composed of the brain and the spinal cord. Everything else, including the autonomic nervous system, is considered part of the peripheral nervous system. An acute event, usually traumatic, can occur in the central nervous system, the most common of which is an ischemic cerebrovascular accident. The next most common type is a traumatic brain injury, followed by traumatic spinal cord injury.
An acute event occurs that causes damage somewhere in the central nervous system. The damage is not ongoing. It's a static event that causes primary cell death and some secondary damage, but it's not progressive. You could contrast that to Parkinson's disease, which is a progressive disease (i.e., the damage keeps occurring). The central nervous system disorders that occur following an acute event usually are more of a static lesion. It occurs once; it's not going to keep occurring over time.
After a central nervous system injury, there is a series of events that occur (Figure 2). If a person receives a blow to the head, for example, that will result in a central core of cell death. That person will suffer primary cell death. In addition to that primary cell death, those neurons that are in communication with those now dead cells are very susceptible to secondary injury. The areas adjacent to initial injury that may die secondarily is called the penumbra. In fact, any cell that communicates with that core area is susceptible to later cell death. Any cell, whether it's in the other hemisphere of the brain or if it's further down in the spinal cord, is susceptible to cell death. Those interconnected areas that may eventually die are called the diaschisis.
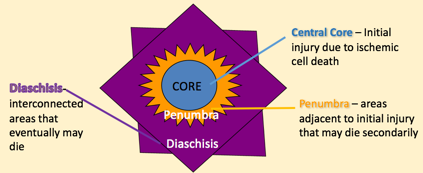
Figure 2. Events after a CNS injury.
Even if the initial area of core cell death is relatively small, that area grows over time. The penumbra is the area that's adjacent to the initial injury; because of the interconnectivity in the central nervous system, a significant lesion may grow from the initial central core death. This is not only true in the brain, but also true in a spinal cord injury. A traumatic injury may cause a central core, but over time (hours or days), that lesion could grow significantly.
The traumatic event causes an area where some cells are not getting the oxygen that they need. The trauma also causes the release of excitatory amino acids, which activate receptors that cause a major influx of calcium. All of this leads to excitotoxicity. A further cascade of events occurs that lead to inflammation in the area of injury, all of which leads to further cell death through necrotic pathways and apoptotic cell death, which causes an increase in the lesion's size. As the lesion size increases, it leads to an increase in the patient's functional deficits. You might start with a small lesion and end up with a very large lesion and a lot of functional deficits.
The pharmacological management after these central nervous system acute injuries is to try to decrease the excitotoxicity. If we can decrease this excitotoxicity and decrease the inflammation, we won't cause growth in the lesion and we'll have fewer functional deficits. That's the pharmacological premise in trying to give drugs that will help to decrease that secondary cell death.
Next, we will review some different diagnoses and take a look at some of the medications that are used for each diagnosis.
Acute Spinal Cord Injury
Research conducted in the 1990s by Bracken, et al in the New England Journal of Medicine looked at using a glucocorticoid (a steroid) in the acute management of spinal cord injury. They particularly looked at methylprednisolone (MP). This study showed such a significant improvement in those patients who were acutely given MP that they stopped the clinical trial, and this was implemented across the country as the standard procedure of giving a high dosage bolus IV of MP, followed by an infusion over the next 23 hours. It was found that this significantly decreased the inflammatory effects and decreased the secondary cell death. They also found that this must be given very early. The maximum window of opportunity is within the first eight hours after a spinal cord injury.
Acute Traumatic Brain Injury
Initially, glucocorticoids and MP were also considered for use with cases of acute TBI. Although it showed great promise in animal models, it led to a higher mortality rate in humans. The appropriate pharmacological mechanism to prevent secondary damage after acute TBI is a little bit different than that of acute SCI. After a TBI, the two main pharmacological goals are to maintain an optimal blood pressure and to normalize intracranial pressure. After a TBI, you don't want the blood pressure hypertensive (too high), but it's equally if not more important to not have the blood pressure get too low. If the blood pressure is too low, the brain is not getting the perfusion that it needs, leading to increased cell death. Research has shown that for every 10-point increase in systolic blood pressure, there is a decrease of almost 20% in the adjusted odds of death. It's very important to maintain normal blood pressure so that we can have appropriate perfusion in the brain. The most common medication used for that purpose is a sympathomimetic drug, phenylephrine HCL, which actually increases blood pressure. For those patients whose blood pressure is low, they will administer this medication. It increases peripheral vascular resistance in the periphery, but it does not do that in the blood vessels, and so we get better perfusion in the brain. It's administered intramuscularly or through IV.
With acute TBI, we also want to maintain normal intracranial pressure (ICP). Due to the nature of a TBI, an increase of intracranial pressure is very common, which can result in significantly further brain damage. The two agents that are most commonly used to control ICP are osmotic agents and barbiturates. Mannitol is the gold standard for normalizing ICP, keeping it at less than 15 mm Hg. Mannitol is an osmotic diuretic that has an effect on intracranial pressure. It enhances cerebral blood flow and brain metabolism. Initially, it can be a good medication to lower intracranial pressure. Use of Mannitol does require an intact blood-brain barrier. As such, it may not be appropriate to use with someone who has sustained significant head trauma. One of the side effects of Mannitol is pulmonary edema, which can later predispose the patient to pneumonia, hypotension and renal injury, as it is metabolized through the kidneys.
It should be noted that there has not been a large randomized controlled trial of Mannitol against a placebo because to do so would be to withhold appropriate treatment for patients with TBI.
Barbiturates are another common classification of medication used to lower the intracranial pressure, by altering the vascular tone. Barbiturates are a potent GABA facilitator. GABA is an inhibitory neurotransmitter. By inhibiting excitatory neurotransmission, barbiturates have the effect of suppressing brain activity. Suppression of brain activity will also lead to sedation, amnesia, and loss of consciousness. As such, barbiturates are used to initiate a medically induced coma. There are two medications (Pentobarbital and Secobarbital) that are frequently used for this purpose. Pentobarbital is short-acting and Secobarbital is medium-acting. Once these barbiturates do get into the system, it takes quite a while for them to be cleared, and for a patient to wake up from a medically induced coma.
Acute Ischemic Stroke
First, I want to differentiate between an ischemic stroke and a hemorrhagic stroke. An ischemic stroke occurs as a result of an obstruction within a blood vessel supplying blood to the brain. If no blood can get through, there is a hypoxic insult and some brain tissue dies. With a hemorrhagic stroke, a weakened blood vessel ruptures, resulting in hypoperfusion. Blood spills into or around the brain and creates swelling and pressure, damaging cells and tissue in the brain. The treatment for ischemic stroke is very different from hemorrhagic stroke. In fact, treatment for an ischemic stroke, if given to someone who is having a hemorrhagic stroke, will usually be fatal. The first step in the emergency room is to differentiate whether the patient is having an ischemic stroke or a hemorrhagic stroke.
For an ischemic stroke, blood pressure control is very similar to TBI, except that patients with an ischemic stroke often present with hypertension and not low blood pressure. Secondarily, they want to break down any clot that is causing the blood vessel occlusion using fibrinolytic drugs, and then give anticoagulants to prevent further damage. By doing that, that will help us to save and prevent secondary damage and save that penumbra and the diaschisis, so the patient has minimal or no functional deficits.
Fibrinolytic agents (commonly known as "clot busters") need to be administered within three hours of symptom onset. There are two fibrinolytic agents that are used frequently: streptokinase and tissue plasminogen activator (tPA). Tissue plasminogen activator is the more expensive of the two drugs. Streptokinase is a lot less expensive, however, it has a shorter shelf life. Hospitals have found that if streptokinase sits on their shelf, it expires and has to be discarded. tPA has a longer shelf life. Even though it's more expensive, they don't need to waste it as often. For that reason, more facilities use tPA than use streptokinase. The mechanism of action of both of these drugs is to activate plasminogen bound to fibrin. It breaks up a clot, which is what's usually causing the occlusion in the blood vessel. The fibrinolytic agents can be given through an IV. They also have been given directly in the brain, which requires a specialized facility.
The second line of defense to minimize secondary damage is to prevent further clots from forming, and that can be done through the use of anticoagulants. One of the most common anticoagulants administered parenterally (through an IV) is heparin. The most common drugs administered subcutaneously are Lovenox and Arixtra. Orally, the most common anticoagulant drug used is a vitamin K antagonist known generically as warfarin, or under the brand name, Coumadin.
Rehab Implications
Medications that are given acutely to prevent secondary damage do have some rehab implications. Acutely, you probably won't be working with a patient until they're deemed medically stable. If you are a working with a patient who may not be medically stable, you likely will be doing some passive, dependent activities (e.g., turning, maintaining mobility, working with respiration, preventing skin breakdowns).
Online Tools
One of the things that I want you to do is to become familiar with some of the online tools at your disposal. A few websites that I really like are PDR.net, MedScape.com, and WebMD.com. There are others, but you need to find websites that you like. Please keep in mind that I'm not officially endorsing any particular website.
A common subacute anticoagulant that can be used with CVA, but it also is often used with orthopedic patients after surgery, is Lovenox. You may want to know if Lovenox has any interactions with other medications. On PDR.net, if you type in the medication name, you can click on the drug summary. For this particular drug, there is a boxed warning, which would also appear on the package insert for the drug. It is named as such because the U.S. Food and Drug Administration requires that it be formatted with a "box", or border around the text. Also on the website, you can see the class of the drug. Lovenox is a fractionated heparin. This is very similar to IV heparin except it can be given subcutaneously. As you scroll down the page on the website, you can find out all kinds of information about the drug. If you're in a hurry and you want to know something, in particular, you can just go to that particular category (e.g., drug interactions, administration instructions, etc.).
You may want to do a drug interaction check. I like the drug interaction checker on WebMD.com. Let's say we want to see if Lovenox will interact with a common pain medication, such as Vicodin. When we enter the names of the two drugs, WebMD will inform us that there is a minor interaction where Vicodin can increase the effects of Lovenox. If we were to dig further, we would see that one of the precautions for Lovenox includes bleeding. If our patient falls frequently that could lead to extensive bruising. We would also find that there is a potential additive effect when NSAIDs are combined with narcotics. With further reading, we would also discover that there are rehab implications for the injection site, along with bleeding and easy bruising.
