Editor’s note: This text-based course is an edited transcript of the webinar, Resistance Training and HIIT: Implications for Sarcopenia and Longevity, presented by Scott Cheatham, PT, DPT, PhD, OCS, ATC, CSCS.
Learning Outcomes
After this course, participants will be able to:
- Define sarcopenia and potential risk factors.
- Describe specific clinical assessments and screening procedures for sarcopenia.
- Describe common prevention and intervention strategies for sarcopenia.
- Detail the research evidence regarding physical therapy interventions for sarcopenia.
Introduction
First and foremost, it's crucial to acknowledge that, as professionals who have pursued higher education and obtained our licenses and specialties, we've encountered the term sarcopenia throughout our careers. However, over the past decade, there has been a significant surge in research on sarcopenia, making it a growing concern for older individuals and experts alike. Experts such as Peter Attia, renowned for their expertise in longevity, emphasize the importance of combating sarcopenia from a younger age, starting as early as our 20s, 30s, and 40s. Consequently, sarcopenia has assumed a prominent role in discussions surrounding health and longevity.
And who better to tackle this challenge than rehabilitation professionals? Certified athletic trainers, occupational therapists, chiropractors, and physical therapists—our collective expertise in movement makes us uniquely positioned to address sarcopenia. This forms the foundation upon which we'll build our discussion. I'd like us to focus on four key learning outcomes. Firstly, we aim to define sarcopenia and explore potential risk factors. While we commonly understand sarcopenia as age-related muscle loss, its definition has evolved with increased diagnostic precision and varying perspectives from research groups worldwide. We'll delve into these nuances and examine how they intersect with resistance training and HIIT.
Our second learning outcome entails describing clinical assessment and screening procedures. Following this, we'll delve into prevention and intervention strategies within our scope of practice, backed by evidence-based rehabilitation approaches. It's paramount to bring the sarcopenia literature up to date for our audience, providing context for why resistance training and HIIT are frontline defenses against sarcopenia. This understanding can inform our clinical practices, potentially integrating circuit training into treatment regimens and client programming.
In essence, our aim is to equip you with updated knowledge and practical insights to address sarcopenia effectively within your professional domain. Given the enormity of this topic and the vast body of evidence available, I've endeavored to distill the essential findings into a concise format for our discussion today. I've organized our session into six modules, each addressing key aspects of sarcopenia and its management.
Module One will focus on defining sarcopenia and examining related factors, providing a foundational understanding of the condition. In Module Two, we'll delve into patient profile assessment. Moving on to Module Three, we'll explore the fitness interventions available to address sarcopenia. Modules Four and Five will specifically address resistance training and HIIT training, two cornerstone approaches in combating sarcopenia. Finally, in Module Six, we'll touch upon documentation and management considerations. Notably, sarcopenia now has its own ICD-10 code, a crucial detail for billing and administrative purposes. We'll briefly discuss the importance of incorporating this code into clinical practice.
By structuring our discussion in this manner, I aim to provide a comprehensive overview of sarcopenia and equip you with practical insights for its management within your professional context.
Module I: Defining Sarcopenia and Related Factors
Definition
As we embark on Module One, let's begin by defining sarcopenia. Traditionally, sarcopenia has been conceptualized as a form of muscle disease characterized by age-related muscle loss, a definition that remains prevalent across research groups globally. This fundamental understanding underscores the adverse changes in muscle composition and function that accompany aging.
It's noteworthy to acknowledge that while sarcopenia predominantly affects older adults, with the current cutoff for consideration set at age 60, emerging research suggests that its onset may occur at younger ages as well. Consequently, researchers are increasingly exploring the manifestations of sarcopenia across different age groups.
When contemplating prevention strategies, we pivot our focus towards three main characteristics prevalent in both younger and older populations: low muscle strength, low muscle quantity and quality of lean muscle mass, and poor physical strength/performance. It's imperative to recognize the variability in individuals' strengths and functional levels, which can influence the manifestation of these characteristics. (Cruz-Jentoft et al. 2019)
While acknowledging this variability, it's worth noting that certain evidence-based screenings, such as grip strength assessments, offer validated approaches to identifying sarcopenia. Throughout our discussion, we'll explore practical ways to integrate these screenings into clinical practice, facilitating early detection and intervention.
In summary, while the foundational definition of sarcopenia endures, contemporary research delves deeper into additional characteristics and phenotypes, broadening our understanding of this multifaceted condition. Through targeted screenings and interventions, we aim to address sarcopenia comprehensively, catering to the diverse needs of individuals across the lifespan.
Statistics
Delving into the statistics surrounding muscle loss, it's crucial to first recognize the concept of normal age-related muscle decline. Researchers generally agree that individuals experience a gradual loss of lean muscle mass, averaging between 3% to 8% per decade after reaching the age of 30 (Volpi et al., 2004, Yu et al. 2016). This decline tends to accelerate after the age of 60, marking a pivotal threshold for the onset of age-related muscle loss. Consequently, age 60 often serves as the cutoff point for considering individuals as potentially affected by sarcopenia. However, it's noteworthy that regular engagement in resistance training and exercise can mitigate the rate of muscle mass loss, underscoring the importance of physical activity in preserving muscle health as we age.
Moving forward, recent research has highlighted another critical milestone: the age of 70. Beyond this age, there is a notable acceleration (15% per decade) in both muscle and weight loss (Yu et al. 2016). This finding underscores the urgency of proactive intervention strategies, particularly for individuals approaching or surpassing this threshold.
A key aspect to bear in mind is the composition of muscle fibers lost during age-related muscle decline. Notably, a higher proportion of type II muscle fibers, known for their fast-twitch capabilities, are typically affected. There are 10% to 40% smaller type II fibers in elderly individuals when compared to younger people (Grimby & Saltin, 1983, Cannataro et al.2021, Milijkovic et al. 2015). This has significant implications for mobility and functionality, as individuals may experience diminished reaction times and reduced capacity for explosive movements. Consequently, interventions targeting the preservation and strengthening of type II muscle fibers, such as resistance training and high-intensity interval training (HIIT), are paramount for maintaining functional independence and quality of life.
Considering worldwide estimates, epidemiological data on sarcopenia remain somewhat limited. However, a study by Yuan and Larsson in 2023 estimated that approximately 10% to 16% of elderly individuals worldwide are affected by sarcopenia. Moreover, the prevalence of sarcopenia appears to be roughly equal between biological males and females, with estimates hovering around 10% for each gender (Shafiee et al.2017).
In summary, while age-related muscle loss is a natural part of the aging process, its progression can be influenced by various factors, including comorbidities and sedentary lifestyles. Of particular concern is the loss of type II muscle fibers, which can significantly impact functional abilities including reaction and gait as individuals age. Proactive engagement in resistance training and exercise represents a critical strategy for preserving muscle health and mitigating the effects of sarcopenia, ultimately promoting greater longevity and quality of life.
Timeline
Understanding the historical timeline of sarcopenia sheds light on its evolution as a recognized medical condition. Dr. Rosenberg's seminal work in 1989 marked the first formal introduction of the term "sarcopenia," derived from the Greek roots signifying "tissue wasting." This conceptualization drew attention to the phenomenon of decreased muscle mass and strength observed in aging individuals.
In 2010, the European Working Group on Sarcopenia in Older Patients (EWGSOP) played a pivotal role in refining Rosenberg's initial definition. By convening a panel of experts, the EWGSOP established a more standardized framework encompassing formal definitions and screening protocols for sarcopenia. This milestone represented a significant step towards achieving consensus within the medical community.
Subsequently, in the years leading up to the COVID-19 pandemic, further advancements in the understanding of sarcopenia emerged. In 2019, the European group, alongside counterparts such as the Asian Working Group on Sarcopenia and the American Sarcopenia Definition and Outcomes Consortium (SDOC), introduced updated definitions and criteria for diagnosing sarcopenia. This ongoing collaboration among international experts aimed to harmonize approaches to sarcopenia assessment and management.
Despite the existence of multiple definitions and criteria, the EWGSOP's guidelines, particularly the EWGSOP2 criteria introduced in their latest update, hold significant sway within the research community. As such, studies exploring sarcopenia often adhere to the standards set forth by the EWGSOP, facilitating a cohesive evidence-based approach to understanding and addressing this condition.
For those inclined towards scholarly inquiry, it's important to recognize the prominence of the European and Asian definitions in the literature, alongside the American perspective offered by the SDOC. While nuances may exist between these frameworks, they generally align, offering a unified foundation for research and clinical practice.
In essence, the historical trajectory of sarcopenia underscores the ongoing efforts to refine our understanding and approach to this complex condition. By acknowledging and embracing the contributions of various international groups, we move closer to achieving consensus and optimizing outcomes for individuals affected by sarcopenia.
Sarcopenia Classification
The classification of sarcopenia into distinct subcategories offers valuable insights into its etiology and implications for clinical practice. Among these classifications, primary sarcopenia stands out as a hallmark of age-related muscle decline, serving as a key diagnostic criterion. No other contributing factors are identified. However, the emergence of secondary sarcopenia underscores the multifactorial nature of muscle loss, with various external factors contributing to its onset.
Secondary sarcopenia arises from a myriad of factors, including poor dietary habits leading to malnutrition, sedentary lifestyles devoid of physical activity, and the presence of comorbidities such as obesity or diabetes. Notably, the use of weight loss medications, such as GLP-1 agonists like Ozempic and Rybelsus, has drawn attention to the potential for medication-induced muscle loss, highlighting the importance of lifestyle modifications in conjunction with pharmacological interventions. Clinicians, particularly those in concierge models of care, are increasingly encountering cases of secondary sarcopenia among patients undergoing weight loss treatments, necessitating a comprehensive approach to mitigate muscle loss and optimize health outcomes, including lifestyle modification such as physical activity, resistance training, and behavior changes such as better dietary habits.
The recognition of secondary sarcopenia as a significant concern within clinical practice underscores the imperative for interdisciplinary collaboration. In cases where patients are undergoing surgical interventions, coordination between physical therapists and orthopedic surgeons becomes paramount to address nutritional considerations and optimize postoperative healing. By aligning treatment strategies with the broader healthcare team, clinicians can better address the complex interplay of factors contributing to sarcopenia and facilitate holistic patient care.
Further subclassifications of sarcopenia, such as acute and chronic forms delineated by timeframes of six months, provide additional granularity for understanding the progression and management of the condition. Acute sarcopenia is less than six months and is often related to acute illness or injury. Chronic is more than six months and is often related to chronic, progressive illness and related to morbidity. These classifications, as outlined in comprehensive guidelines such as those developed by Cruz and Jentoft (2019), serve as valuable resources for clinicians navigating the complexities of sarcopenia diagnosis and treatment.
The recognition of secondary sarcopenia as a distinct entity underscores the importance of addressing underlying factors beyond age-related changes alone. By adopting a multidisciplinary approach and leveraging evidence-based guidelines, clinicians can effectively identify and manage sarcopenia across its various presentations, ultimately promoting optimal patient outcomes and quality of life.
Scientific Societies Studying Sarcopenia
The landscape of sarcopenia diagnosis and classification is shaped by the contributions of several prominent scientific societies and organizations, each offering unique perspectives and criteria for assessment. Among these, the European Working Group on Sarcopenia in Older People (EWGSOP), the Asian Working Group for Sarcopenia (AWGS), the American Sarcopenia Definitions and Outcomes Consortium (SDOC), and other workgroups including the Foundation for the National Institutes of Health (FNIH), International Working Group on Sarcopenia in Older People (IWGS), and Baumgartner, Delmonico, and Morley represent key entities driving advancements in sarcopenia research and clinical practice. (Stuck et al., 2023).
Upon closer examination, it becomes evident that there is significant overlap in the criteria proposed by these groups, with common themes such as low muscle strength, diminished muscle quantity and quality, and impaired physical performance emerging as central diagnostic indicators, as seen in Figure 1. However, nuanced differences in emphasis exist, with the American group prioritizing measures of muscle strength and physical performance, while the European group adopts a more comprehensive approach encompassing multiple facets of muscle health.

Figure 1. Major scientific society sarcopenia definitions. (Click here to enlarge this image.)
As a researcher and practitioner, I hold a preference for the European guidelines due to their holistic and pragmatic approach to sarcopenia assessment. Figure 2 shows their simplified diagnostic criteria, which categorize sarcopenia into probable and severe forms based on combinations of low muscle strength, quantity, and quality and offer a practical framework for screening and evaluation.
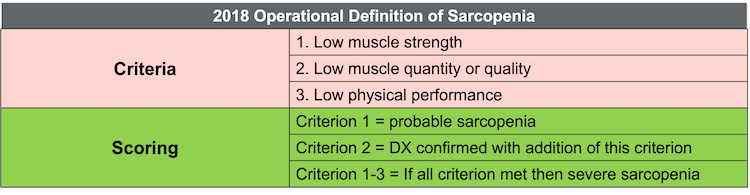
Figure 2. EWGSOP 2018 operational definition of sarcopenia. (Click here to enlarge this image.)
It's crucial to contextualize these diagnostic criteria within the broader clinical landscape, recognizing that individual variability and unique circumstances may influence interpretation. For instance, transient sarcopenia may occur in patients undergoing significant periods of immobilization or recovery from severe illness or injury. In such cases, the diagnostic criteria serve as valuable screening tools rather than definitive diagnostic markers.
The evolving understanding of sarcopenia underscores its significance as a predictor of mortality and overall health outcomes. As healthcare providers, it's incumbent upon us to remain vigilant in identifying and addressing sarcopenia, recognizing its implications for morbidity and mortality. By embracing evidence-based guidelines and advocating for proactive interventions such as resistance training and physical activity, we can empower individuals to combat sarcopenia and optimize their muscle health throughout their lifespan.
Related Factors and Consequences
Examining the related factors and consequences of sarcopenia illuminates the interconnectedness of various physiological and lifestyle factors contributing to its onset. Obesity, physical inactivity, malnutrition (low protein), cigarette smoking, and even extreme sleep durations emerge as notable risk factors predisposing individuals to sarcopenia. While some factors, such as obesity and physical inactivity, are intuitive in their association with muscle loss, others, like extreme sleep duration, offer intriguing insights into the multifaceted nature of sarcopenia risk.
Moreover, age-related declines in androgen and growth hormone levels, along with insulin resistance, further exacerbate the risk of sarcopenia, reflecting the intricate interplay between hormonal regulation and muscle health. Notably, reductions in type II muscle fibers and blunted muscle protein synthesis responses to protein intake and resistance training underscore the physiological underpinnings of sarcopenia development, highlighting the importance of lifestyle interventions in mitigating its effects. (Yuan & Larsson, 2023)
As individuals age, the cumulative impact of these risk factors contributes to a gradual decline in muscle mass and function, underscoring the imperative for proactive intervention strategies. Indeed, resistance training emerges as a cornerstone approach in addressing sarcopenia risk factors, offering a viable means of preserving muscle mass and optimizing physiological function. By advocating for regular physical activity, balanced nutrition, and targeted interventions to address hormonal imbalances and metabolic dysregulation, healthcare providers can empower individuals to mitigate the risk of sarcopenia and maintain optimal muscle health throughout their lifespan.
Exploring the associated factors linked to sarcopenia unveils a complex interplay of physiological, lifestyle, and medical variables that contribute to its development. Chronic diseases such as COPD, CHF, CKD, diabetes mellitus, HIV, and cancer emerge as significant risk factors, reflecting the systemic impact of underlying health conditions on muscle health. Additionally, the concomitant use of medications further complicates the picture, potentially impacting nutrient absorption and appetite regulation.
There is a spectrum of associated factors for sarcopenia encompassing aging, vitamin and trace element deficiencies, changes in hormone levels, chronic inflammation, osteoporosis, anemia, and malnutrition. These factors collectively underscore the multifaceted nature of sarcopenia etiology, highlighting the intricate interplay between systemic health, hormonal balance, and nutritional status.
Of particular concern are lifestyle factors and medication effects that may exacerbate sarcopenia risk. For instance, the use of weight loss medications, such as GLP-1 agonists, in the absence of lifestyle modifications may predispose individuals to malnutrition, anemia, and chronic inflammation, thereby amplifying the risk of sarcopenia.
While addressing each associated factor comprehensively within clinical practice may prove challenging, collaborative approaches involving multidisciplinary healthcare providers offer a viable solution. Referral to registered dieticians for nutritional counseling and education can complement existing interventions, providing individuals with tailored strategies to optimize dietary choices and mitigate sarcopenia risk. By leveraging collaborative networks and adopting a holistic approach to patient care, healthcare providers can address the diverse array of factors contributing to sarcopenia, ultimately promoting optimal muscle health and overall well-being.
Exploring the related consequences of sarcopenia sheds light on the profound impact of muscle loss on various aspects of health and well-being. Sarcopenia is intricately linked to a host of adverse outcomes, including mortality, cognitive impairments, osteoporosis, falls, fractures, functional decline, and hospitalizations. These consequences stem from the fundamental role of muscle strength and function in supporting mobility, balance, and overall physical resilience.
Additionally, sarcopenia exhibits associations with metabolic syndrome, diabetes, non-alcoholic liver disease, liver fibrosis, hypertension, depression, and dysphagia, as evidenced by correlational studies examining the intersection between muscle health and systemic health parameters. However, it's crucial to interpret these associations within the context of correlational research, recognizing that causality cannot be inferred definitively from observational data alone.
Delving into the pathophysiology of sarcopenia unveils a complex interplay of age-related changes encompassing age-related declines in anabolic hormones, age-related neurodegeneration of type II fibers, age-related increase in inflammatory markers, changes in muscle fiber quality, mitochondrial dysfunction, and reductions in satellite cell populations. This multimodal aging process underscores the systemic nature of sarcopenia, implicating diverse physiological systems in its etiology.
The recognition of sarcopenia as a multifaceted condition with far-reaching implications underscores the importance of proactive intervention strategies aimed at preserving muscle mass and function. By promoting physical activity, resistance training, balanced nutrition, and targeted interventions addressing hormonal imbalances and inflammatory processes, healthcare providers can mitigate the risk of sarcopenia and its associated consequences, ultimately enhancing the quality of life and promoting healthy aging.
Bottom Line
A comprehensive understanding of sarcopenia, encompassing its definitions, classifications, historical context, screening procedures, risk factors, consequences, and pathophysiological mechanisms, is a foundational pillar for effective treatment planning and intervention. The European Working Group on Sarcopenia in Older People seems to be the most widely used classification and criteria. By delving into the nuances of different working group definitions and theoretical frameworks, healthcare professionals can elucidate the rationale behind targeted exercise programming and therapeutic approaches.
Moreover, recognizing the dynamic nature of sarcopenia research underscores the importance of ongoing education and adaptation within clinical practice. As new insights emerge and evidence accumulates, healthcare professionals must remain vigilant, continuously updating their knowledge base to deliver optimal care to their clients.
Ultimately, by integrating a robust understanding of sarcopenia into clinical practice, healthcare professionals can tailor interventions to address each individual's unique needs and challenges, thereby promoting optimal muscle health, functional independence, and overall well-being.
Things to think about:
- Professionals should have an understanding of the different classifications for sarcopenia.
- The European Working Group on Sarcopenia in Older People seems to be the most widely used classification and criteria.
- Professionals should have a strong understanding of sarcopenia risk factors, consequences, and pathophysiology.
Module II: Patient Profile and Sarcopenia Assessment
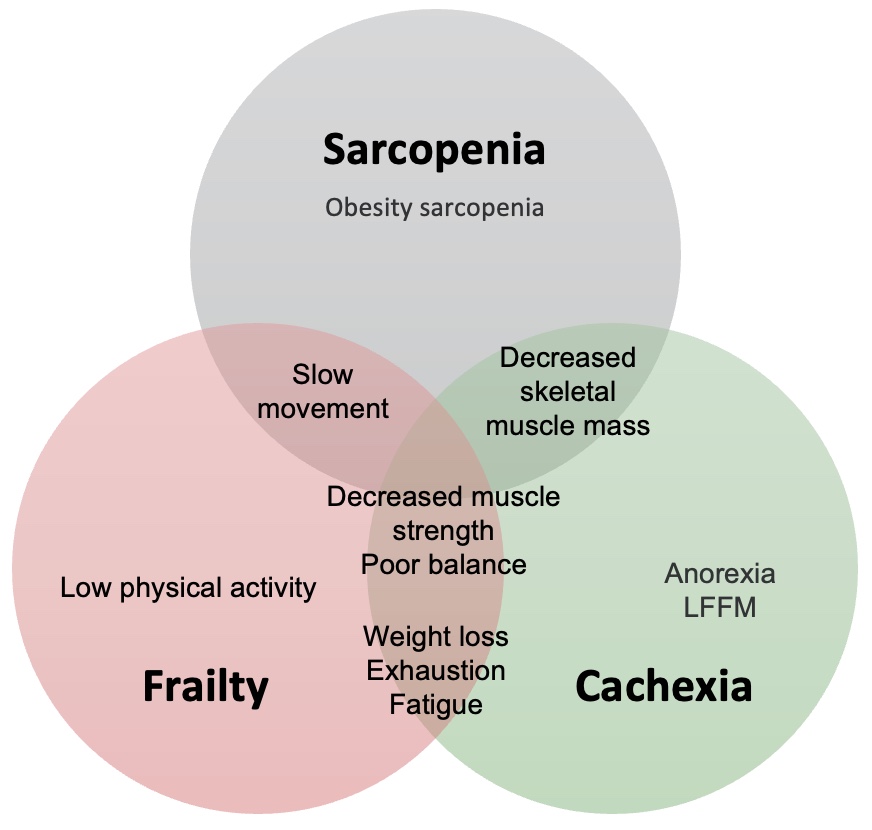
Figure 3. Patient profile and sarcopenia assessment. (Click here to enlarge this image.)
As we get into Module Two, an intriguing aspect surfaces regarding patient profiles: the interconnectedness of sarcopenia with frailty and cachexia. These terms, though familiar to many over the years, are gaining prominence, particularly in the context of older individuals with various health challenges. It's not uncommon to encounter scenarios where individuals, such as former smokers with COPD, exhibit signs of cachexia alongside frailty, characterized by pronounced weakness and advanced physical decline.
Researchers are now considering sarcopenia as an early stage in this progression, akin to osteopenia preceding osteoporosis. This conceptualization underscores the potential of sarcopenia as a harbinger of significant functional decline and deteriorating health. Understanding this trajectory is pivotal, as it enables healthcare professionals to intervene early, mitigating the risk of progression to more severe states.
Frailty, as defined, represents a cumulative decline in multiple body systems ((Fried et al. 2001, Xue 2011). Marked by diminished grip strength, low energy levels, slower walking speed, decreased physical activity, and unintentional weight loss, it mirrors a severe form of sarcopenia. The delineation between these conditions blurs, suggesting a spectrum of sarcopenic states.
Similarly, cachexia shares many attributes with frailty, albeit with a distinct etiology rooted in chronic illness, such as COPD (Evans et al. 2008). It manifests as a complex metabolic syndrome characterized by low muscle strength, weight loss, low energy, low fat-free muscle mass, and abnormal biochemistry. The overlap among these conditions underscores the need for vigilance and comprehensive management strategies.
Viewed as a continuum, sarcopenia may manifest in younger adults as sedentary lifestyles take their toll, progressing to frailty in older age, particularly in the presence of comorbidities. In cases where chronic illness exacerbates the condition, cachexia may ensue, marking a critical juncture in the clinical trajectory. This nuanced perspective empowers healthcare providers to navigate the complexities of sarcopenic conditions, offering tailored interventions to optimize patient outcomes.
Patients Physiological Profile
Considerations:
- Training age: Past & present experience
- Anthropometric: BMI, body composition
- Cardiorespiratory: Estimated or direct V02max
- Muscle strength/power/endurance: UE/LE and core
- Flexibility/mobility: UE/LE and trunk
- Function/Performance: Performance field tests (baseline)
- Psychosocial: Exercise readiness, social support, goals
In my clinical practice, I prioritize monitoring the physiological profile of each patient, particularly when addressing sarcopenia. This module underscores the significance of understanding the profile of individuals affected by sarcopenia and the assessments pertinent to their management. Let's delve into these key assessments:
Firstly, let's discuss training age. This concept, originating from the sports medicine or athletic realm, sheds light on an individual's past and present exercise experience. Consider this analogy: if you have a player who's been in the NBA for 15 or 20 years, like LeBron James, his training age may be 20 years, despite being chronologically younger. Hence, when addressing sarcopenia, we must consider the patient's exercise history, as it reflects their self-efficacy. This experience, which I simply term as training age, guides our approach to programming. For instance, if a patient has a long history of consistent exercise, such as being a former college athlete who has maintained physical activity since, they are akin to a mature athlete. With such individuals, we can streamline our programming efforts without the need for extensive nurturing and education. Additionally, we must also assess their psychosocial and emotional readiness to engage in physical activity.
Moving on to anthropometric data, it's crucial to understand the limitations of BMI (Body Mass Index). While widely used for its convenience in comparing individuals across different populations and healthcare systems, BMI has significant flaws. Therefore, a more accurate assessment lies in body composition analysis. Currently, DEXA scans stand out as the gold standard for this purpose, providing comprehensive insights into lean muscle mass, fat distribution, and appendicular composition. Similarly, bioelectrical impedance analysis, offered through portable devices, offers valuable information on muscle distribution across various body segments. Many therapists in the sports medicine field integrate bioelectrical impedance measurements into their initial assessments.
Cardiorespiratory assessment plays a vital role, especially in designing HIIT (High-Intensity Interval Training) programs. However, it's essential to note that while aerobic activity contributes to overall health and lifestyle modification, it may not be the primary intervention for sarcopenia. Resistance training or HIIT is recommended for loading the system effectively. When incorporating HIIT (High-Intensity Interval Training) into a regimen, it's crucial to conduct thorough assessments.
HIIT typically involves a circuit at various stations performed at maximum effort. Therefore, it's essential to screen the individual's blood pressure and assess their cardiorespiratory function. In clinical settings like mine, where I operate in California, I delegate the three-minute step test to one of my aides, who is a certified athletic trainer and fitness professional. I oversee this process, ensuring accuracy and safety. Once we've completed the three-minute step test, we obtain a baseline estimate of VO2, allowing us to establish different training zones. This approach enables tailored programming suited to the individual's fitness level and goals.
Assessing muscle strength, power, and endurance is essential in developing effective intervention strategies. For muscle strength assessment, I've transitioned to using a handheld dynamometer to gather more precise and scientific data during muscle testing sessions. Muscle power can be evaluated through exercises like medicine ball throws, box jumps, or endurance-based power activities, depending on the individual's capabilities.
Endurance exercises are also integral to the assessment process. While the plank exercise is commonly used to evaluate core endurance, classic endurance tests such as push-ups, crunches, or sit-ups offer valuable insights into overall endurance capacity. Many professionals, including physical therapists, occupational therapists, and chiropractors, rely on these tests for assessment purposes.
Additionally, flexibility and mobility assessments are crucial, especially for older patients with multiple comorbidities and sedentary lifestyles. Assessing posture, flexibility, and mobility helps tailor interventions and avoid potential risks, such as aggravating conditions like upper cross syndrome or forward head posture.
Function and performance tests play a pivotal role in guiding intervention strategies. Depending on the individual's age and activity level, agility tests like ladder drills or traditional assessments such as the bird balance test or Timed Up and Go (TUG) test may be employed.
Furthermore, considering psychosocial factors is essential. Understanding the patient's exercise readiness, social support system, and personal goals can significantly influence intervention planning. A brief conversation can provide valuable insights into their readiness and motivations, allowing for personalized programming aligned with their objectives.
Incorporating these assessments into practice ensures comprehensive care and tailored interventions, promoting improved functional outcomes and overall well-being. Whether these assessments directly align with your practice or serve as informative tools, they offer valuable insights for optimizing patient care.
Patient Subjective
Let's delve into the patient's subjective assessment, a standard practice in our evaluation process. Alongside the intake paperwork, we thoroughly review their complete medical history, including past treatments and current medications, aiming to identify any potential yellow or red flags.
Subjective assessment questions:
- General
- Medical history
- Medical treatment and current medications
- Lifestyle: work, exercise, and dietary habits
- Current lifestyle and fitness goals
- Client Management:
- Current strategy for monitoring the condition
- Current level of physical and functional activity
- MD/PT activity guidelines
We examine various aspects, such as lifestyle and fitness. Additionally, I often encounter individuals who have undergone weight loss and now seek fitness guidance, a common scenario in my concierge practice. However, some may exhibit early signs of sarcopenia due to factors like post-surgery recovery, prolonged sedentary behavior, or illness, including long COVID.
I also inquire about client management strategies for diagnosed conditions and current activity levels. Are they collaborating with other healthcare professionals like doctors or physical therapists? This information provides valuable context for tailoring our approach.
Moving forward, I employ goal-setting techniques, utilizing the SMART criteria—specific, measurable, attainable, realistic, timely, and self-determined. Setting specific and time-bound goals is paramount for individuals engaged in weightlifting or resistance training. Whether it's aiming to increase workout frequency or achieve certain milestones by month-end, clarity and specificity are key.
Furthermore, as healthcare providers, we establish our own short and long-term goals in collaboration with the patient. This collaborative approach ensures alignment between patient aspirations and healthcare objectives, fostering a sense of partnership and empowerment. Fortunately, this process can be efficiently accomplished during the initial session, setting the stage for productive collaboration and progress.
Sarcopenia Screening
Let's transition to a scenario where sarcopenia is suspected. Many healthcare providers are adopting a standardized screening approach to facilitate diagnosis. Recent physical therapy research has highlighted the efficacy of a step-by-step process consisting of four key stages: Find, Assess, Confirm, and Evaluate Severity, as seen in Figure 4. This comes from the European Working Group. Let's delve into each of these steps.
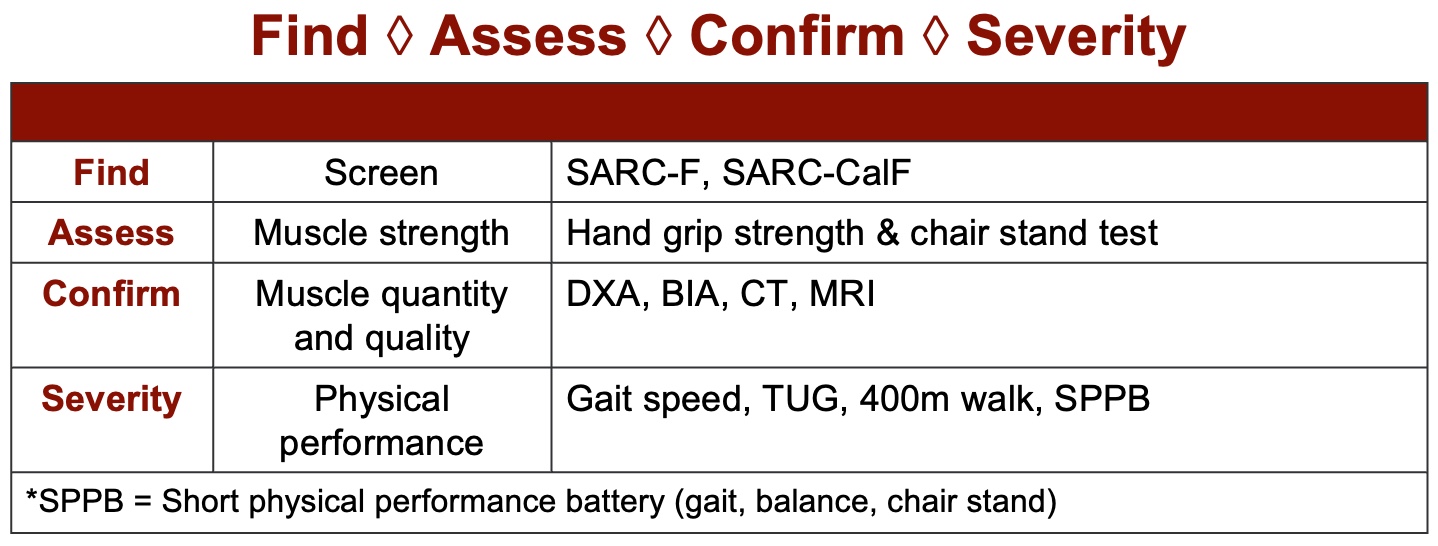
Figure 4. Sarcopenia screening tool. (Click here to enlarge this image.)
The initial step in the process is "Find," which involves the utilization of a screening questionnaire. Notably, this approach is typically applied to older individuals aged 60 or above. While there has been a study involving individuals in their 40s, it's crucial to recognize that the SARC form, commonly used for this purpose, has not yet been fully validated for younger populations, although adaptations have been made.
The next step, "Assessing," revolves around evaluating strength, while "Confirming" entails the utilization of bioelectric or anthropometric measurements. Finally, "Severity" focuses on assessing function. These steps are recommended by the European Working Group and are commonly performed by physical therapists, occupational therapists, and chiropractors.
Assessment: Find
Upon assessment, individuals are typically administered the SARC form, seen in Figure 5, which comprises approximately five questions pertaining to strength, function, and lifting. However, research has revealed that the sensitivity of this form ranges from 20% to 50%, indicating its limited predictive capability. Conversely, its specificity, ranging from 60% to 80%, nearly reaching 90%, suggests a stronger confirmatory aspect.
Originally intended as a confirmatory assessment rather than a screening tool, the SARC form demonstrates good prognostic value when used within the broader context of the European definition of sarcopenia, encompassing factors such as strength and function. Studies, including one by Nishikawa in '21, have underscored the form's utility across various chronic conditions, including Parkinson's, diabetes, chronic liver disease, systemic sclerosis, chronic heart failure, cardiovascular disease, stroke, COVID-19, and gastrointestinal disease.
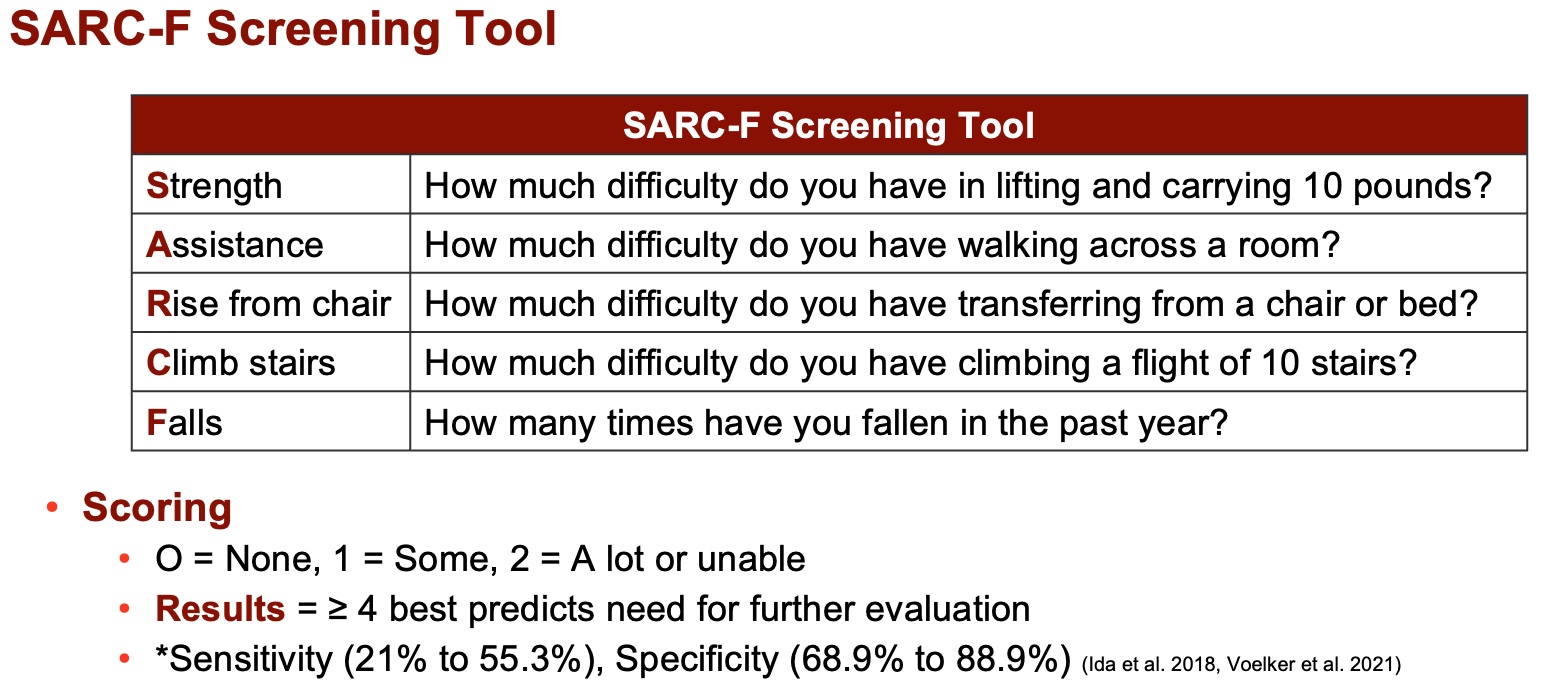
Figure 5. SARC-F screening tool (Nishikawa et al. 2021). (Click here to enlarge this image.)
In response to the SARC form's limited sensitivity, researchers devised the SARC-CalF, which combines the original questionnaire with calf circumference measurements, as seen in Figure 6. Although calf size variation may raise concerns regarding genetic predisposition, this additional criterion aims to enhance specificity rather than sensitivity. The resulting SARC-CalF screening tool, with a cutoff score of 11 points, emphasizes confirmatory aspects without significantly boosting sensitivity.
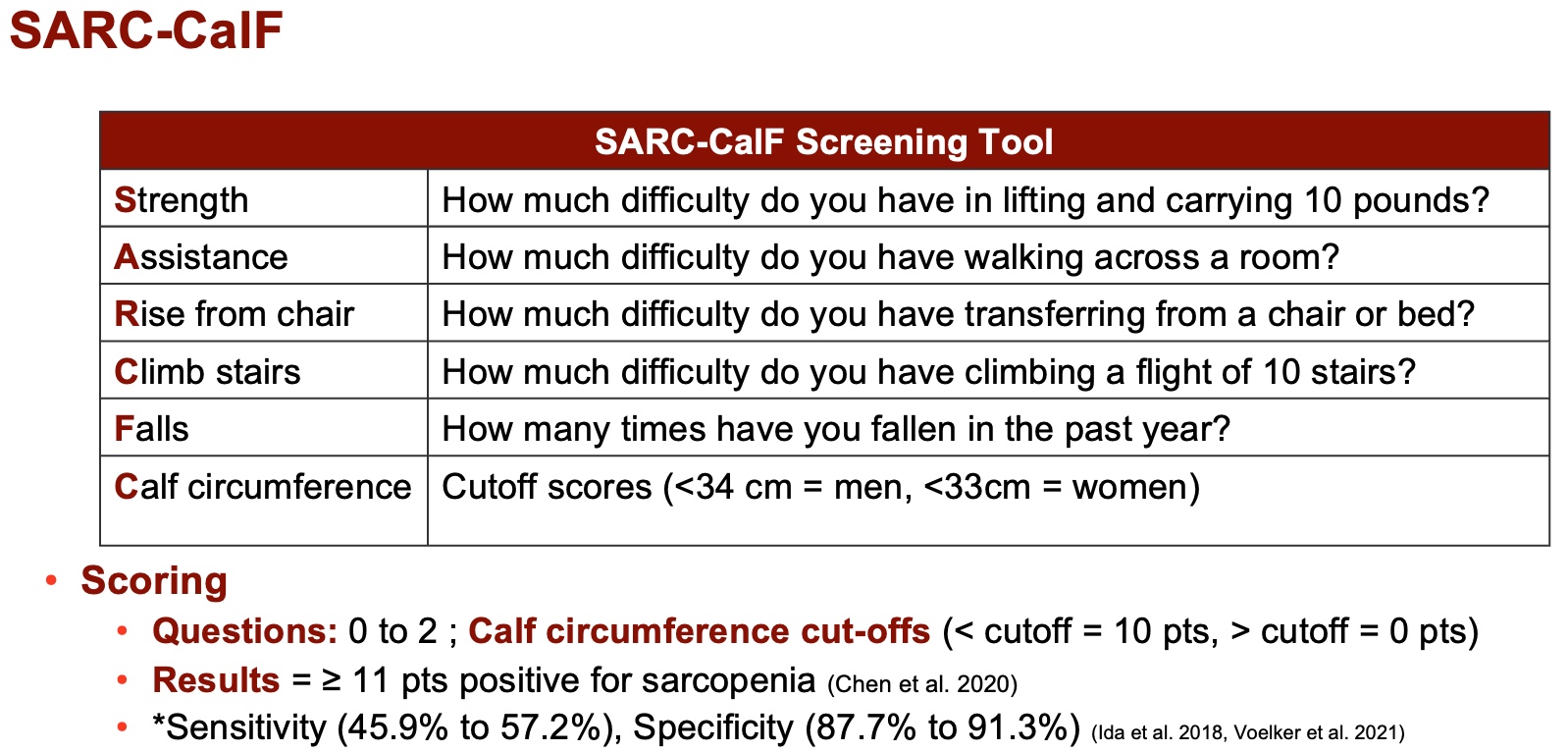
Figure 6. SARC-CalF screening tool (Nishikawa et al. 2021). (Click here to enlarge this image.)
While alternative SARC questionnaires exist, the European group advises combining the original SARC form with complementary measures due to its predominantly confirmatory nature. Therefore, practitioners should approach its utilization with the understanding that it serves as a piece of a broader diagnostic puzzle rather than a standalone screening tool.
Assessment: Assess
This led to the development of an assessment phase focusing primarily on strength.
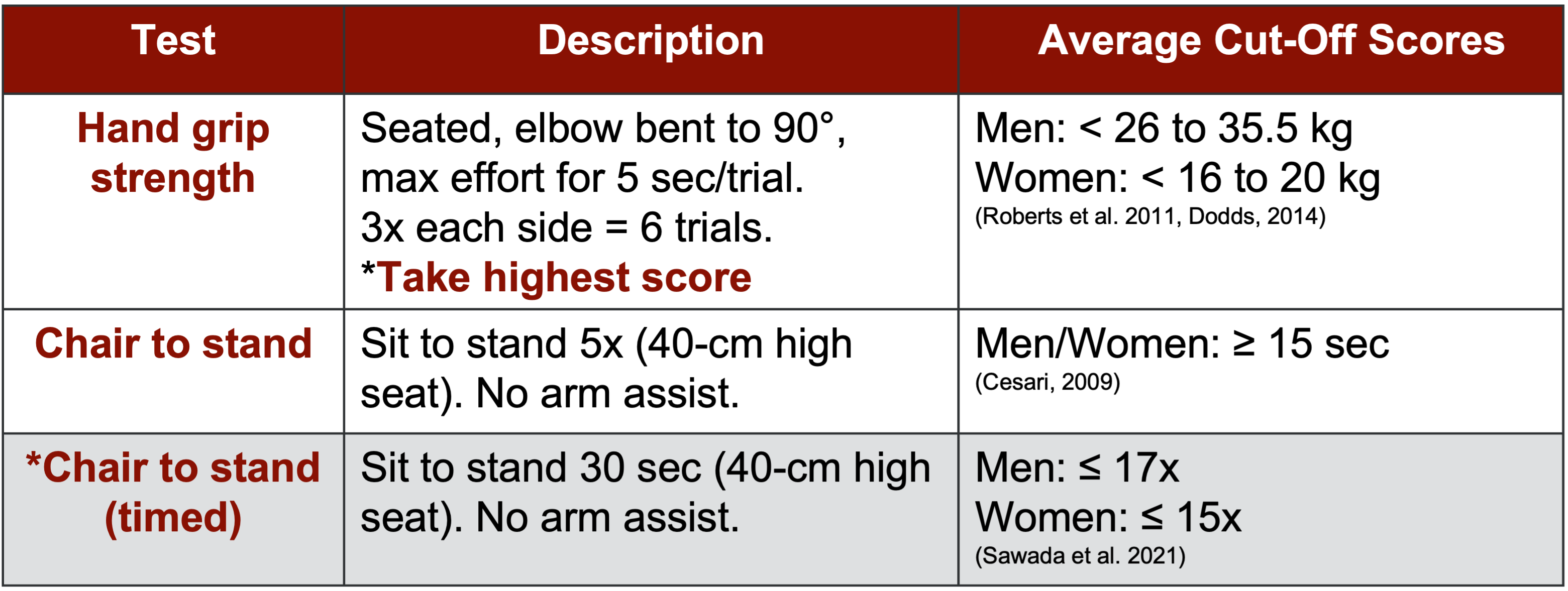
Figure 7. Assessment for strength (Guttikonda & Smith, 2021). (Click here to enlarge this image.)
Grip strength, widely recognized for its applicability in assessing overall function due to the frequent use of hands in daily activities, emerges as a crucial component of sarcopenia evaluation. Additionally, the chair-to-stand test, available in standard and 30-second variations, is another significant assessment tool.
For individuals aged 60 and above who exhibit potential sarcopenic symptoms, the recommended protocol involves initial screening with the questionnaire followed by assessments of grip strength and chair-to-stand performance. As outlined in the diagnostic definition, meeting one, two, or all three of these criteria may lead to a sarcopenia diagnosis.
Notably, hand grip strength holds particular importance, prompting ongoing research to explore its potential as an early indicator of sarcopenia, even in younger age groups. Currently underway, studies targeting individuals in their 20s, 30s, and 40s aim to establish normative data and identify potential precursors to sarcopenia, thereby facilitating early intervention strategies.
As the research group endeavors to advance understanding in this area, the assessment phase underscores the significance of evaluating fundamental aspects of function and strength, laying the groundwork for comprehensive sarcopenia management.
Assessment: Confirm
When it comes to confirming sarcopenia, various imaging and electrical impedance techniques come into play. The DEXA (Dual-Energy X-ray Absorptiometry) scan, Bioelectric Impedance Analysis (BIA), MRI, and CT scan are commonly utilized. BIA, in particular, has shown robust validity in this population, with clinical-grade instruments available at affordable prices, typically ranging from $300 to $400. Incorporating BIA into assessments has proven highly beneficial, often providing patients with insightful printouts or app-based summaries that effectively convey their physiological status.
The confirmatory aspect of screening, in my experience, offers valuable clarity to both healthcare providers and patients alike. For instance, Figure 8 serves as a comprehensive summary, outlining cutoff values derived from DEXA and BIA measurements in alignment with major society definitions. These cutoff values serve as reference points, aiding in the interpretation of assessment results and guiding subsequent interventions effectively.
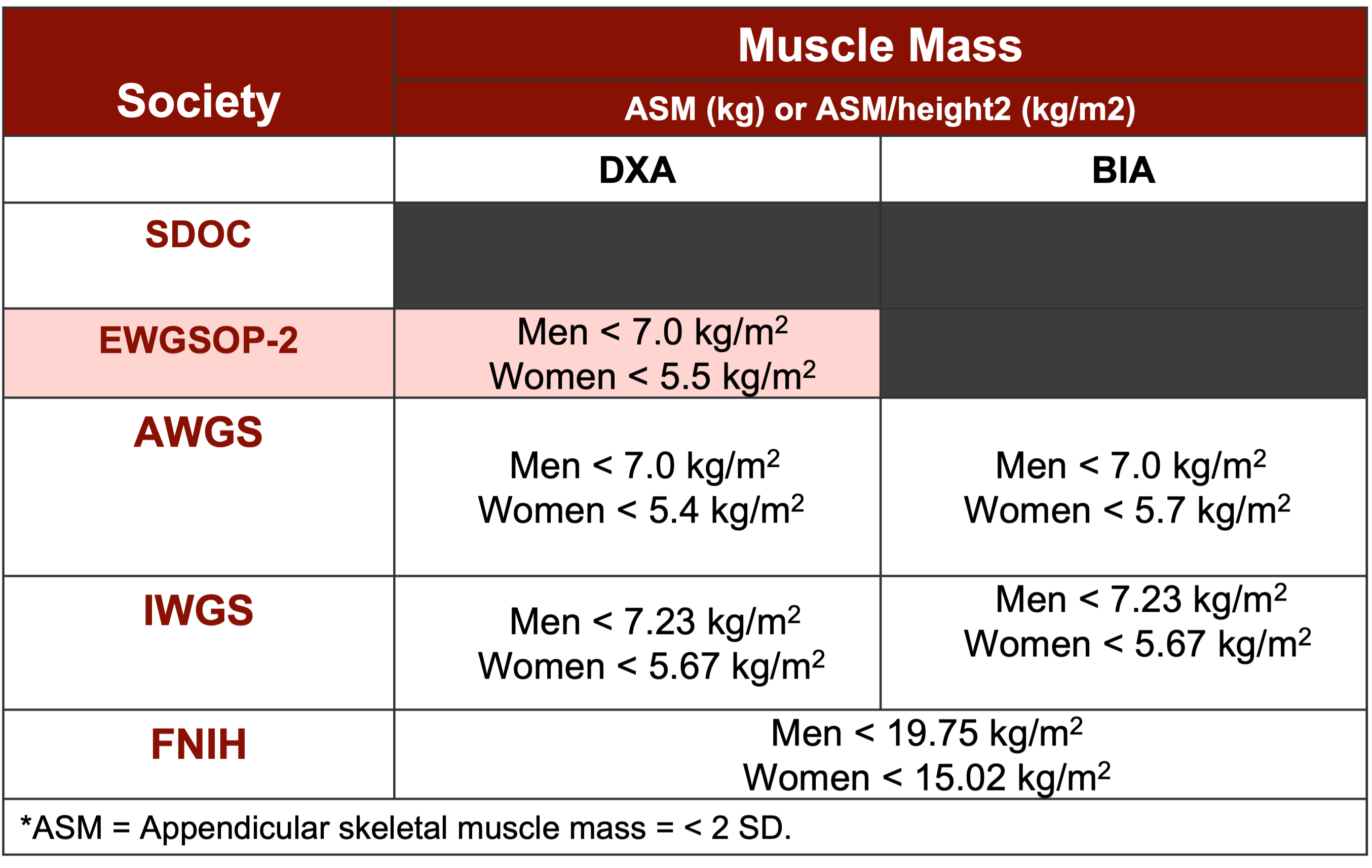
Figure 8. Summary of cutoff values for DXA and BIA (Yuan and Larsson, 2023). (Click here to enlarge this image.)
Assessment: Severity
Regarding assessing the severity of sarcopenia, the European group primarily recommends evaluating gait speed alongside other measures, such as the Short Physical Performance Battery, which combines sit-to-stand, standing balance, and gait speed assessments. Additionally, tools like the Timed Up and Go (TUG) test and the 400-meter walk test are suggested indicators.
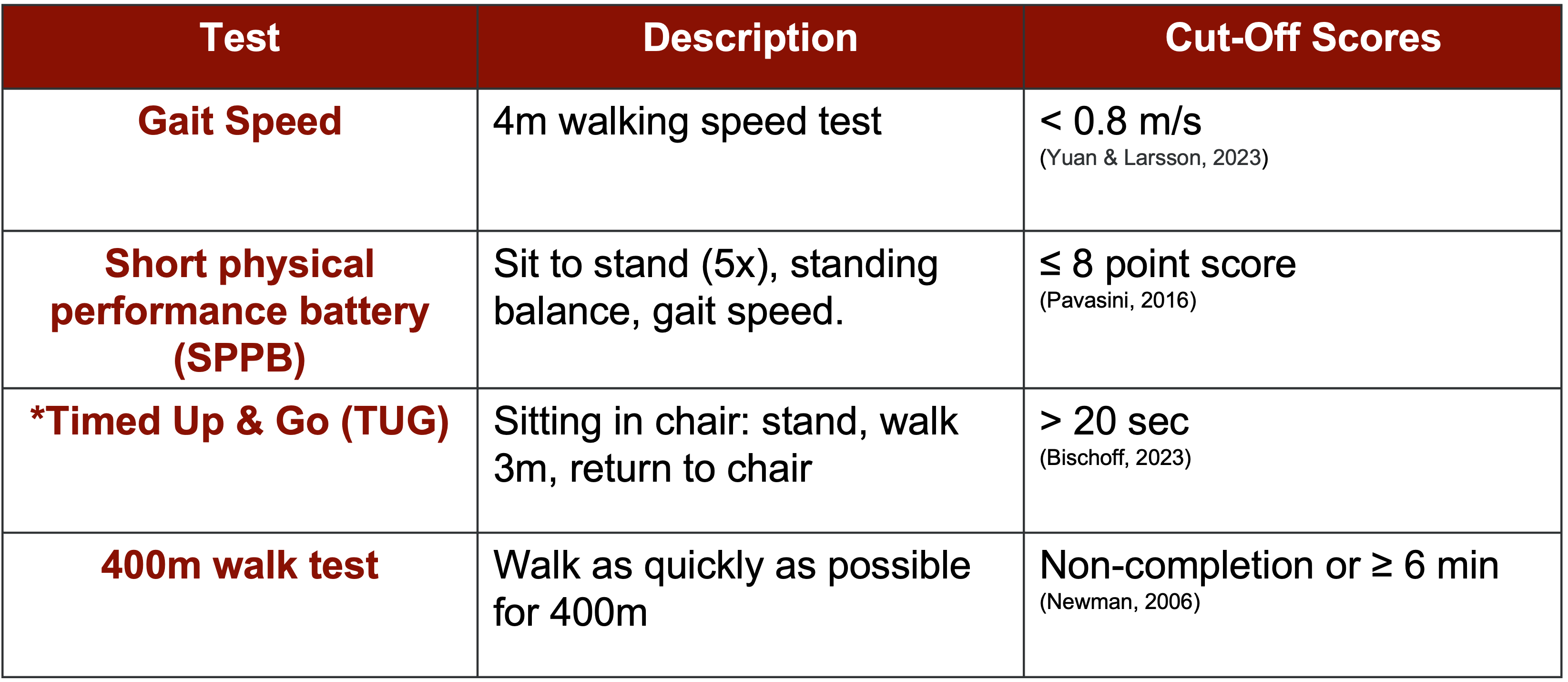
Figure 9. Assessing severity. (Click here to enlarge this image.)
These assessments, detailed in Figure 9, provide cutoff values to aid in diagnosis. For instance, a gait speed below 0.8 meters per second is considered indicative of sarcopenia. This four-step screening process, encompassing find, assess, confirm, and severity, has gained traction in clinical practice, particularly among physical therapists.
Figure 10 offers a comprehensive overview of these assessments, facilitating a holistic understanding of muscle mass, strength, and performance evaluation. The European group's approach emphasizes evidence-based diagnosis, leveraging various assessments and cutoff values to accurately identify sarcopenia.
However, research on pre-sarcopenia remains limited, posing a challenge in identifying precursor states. Efforts are underway to develop screening tools, such as grip strength assessments, aimed at detecting early signs of sarcopenia in younger individuals. This ongoing exploration holds promise for early intervention and management strategies.
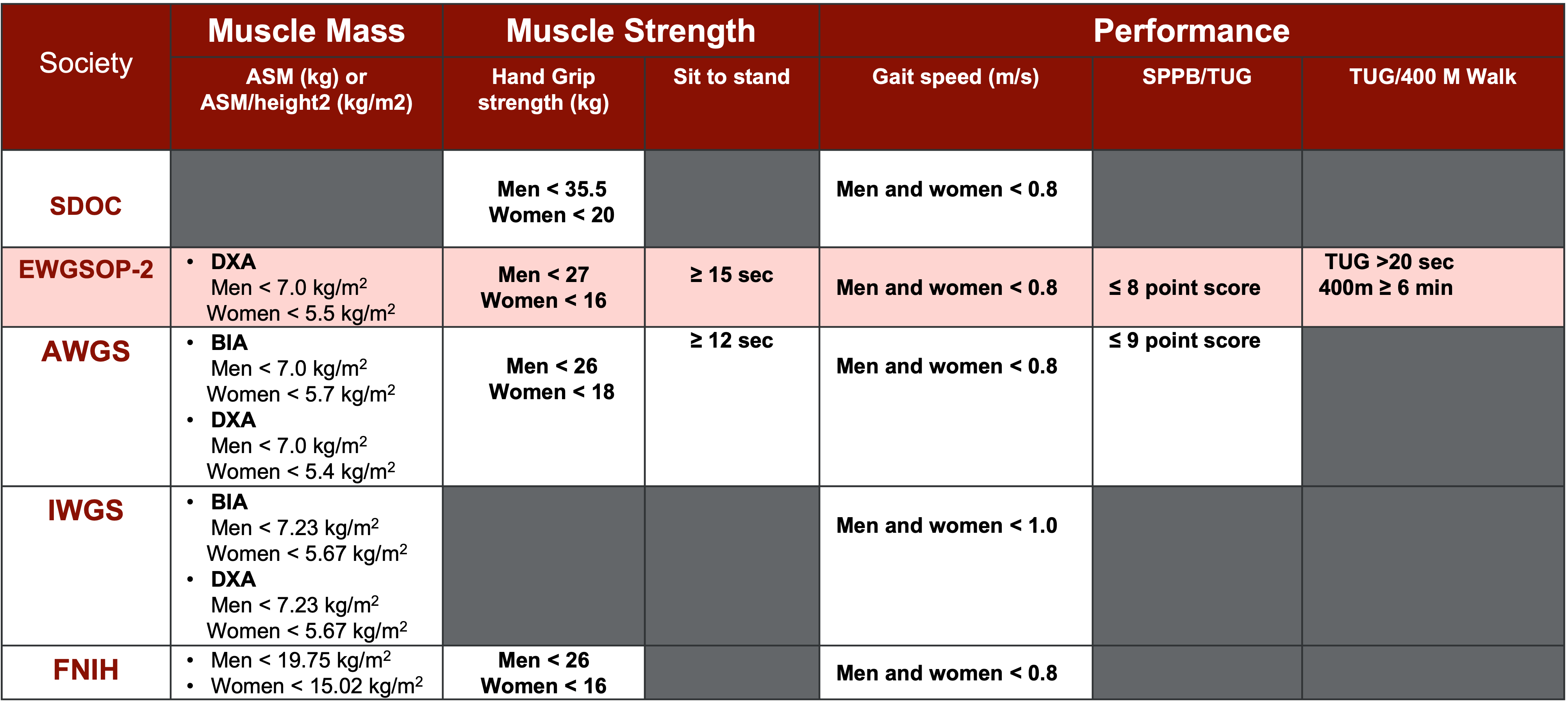
Figure 10. Summary of sarcopenia cut-off scores (Yuan & Larsson, 2023). Note *Appendicular skeletal muscle (ASM)= < 2 SD. (Click here to enlarge this image.)
Other Screening Tools
The Ishii screening tool offers a composite score based on age, hand grip strength, and calf circumference, which are regarded as reliable markers of muscle function and strength. While initially introduced in 2014 and 2016, it gained more attention and validation in subsequent years, particularly in 2019 and 2020. Studies have shown promising sensitivity rates of 85% in biological males and 75% in biological females, with specificity rates of 88% and 91%, respectively. This screening tool shows potential for use in younger populations, although norms are still being established.
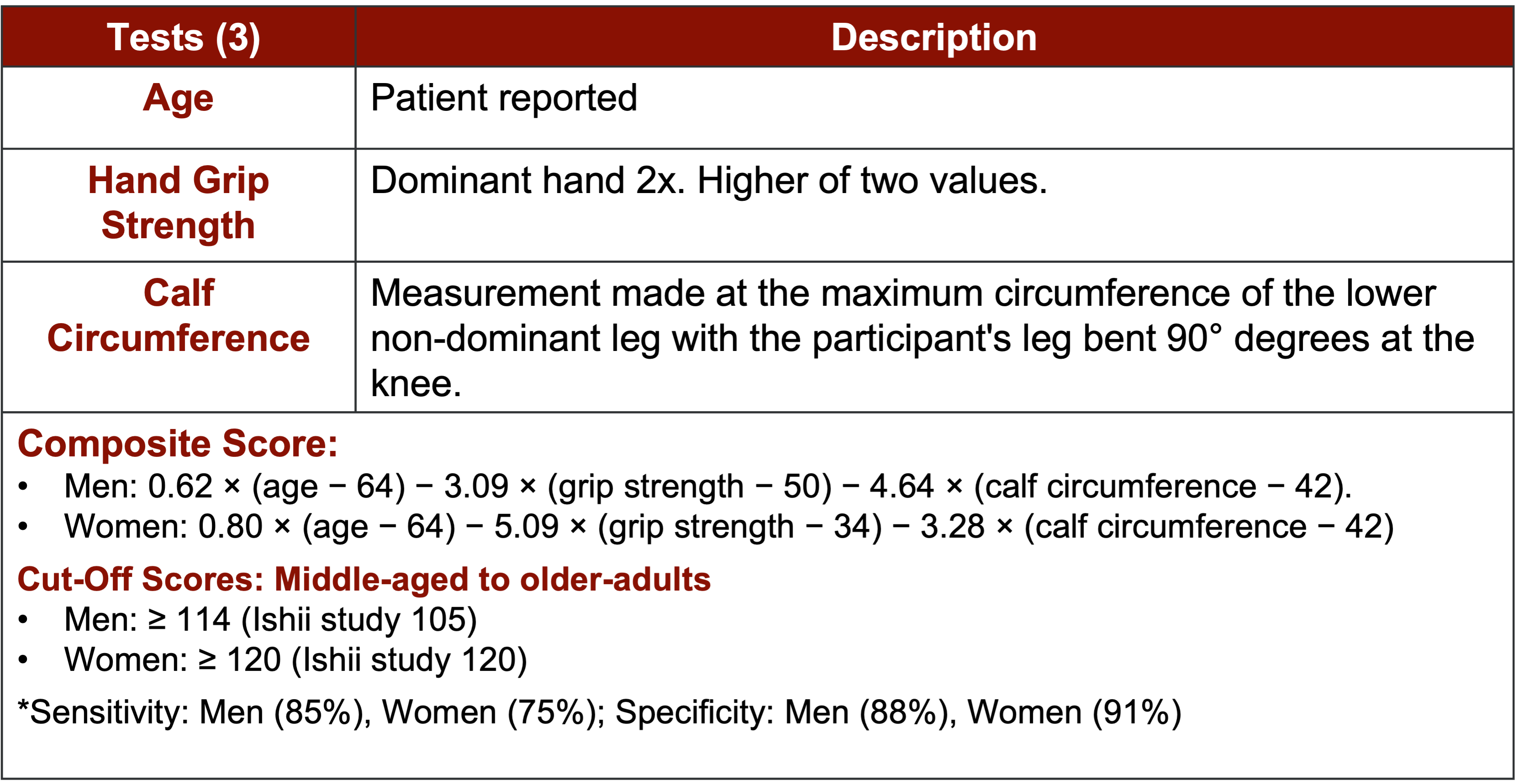
Figure 11. Ishii screen for sarcopenia. (Click here to enlarge this image.)
In addition to the Ishii screening, other tests like the MSRA (Muscle Strength Risk Assessment) and the Short Portable Sarcopenia Measure are available. However, the Ishii and the European screening tools are among the most widely recognized and researched options in the field.
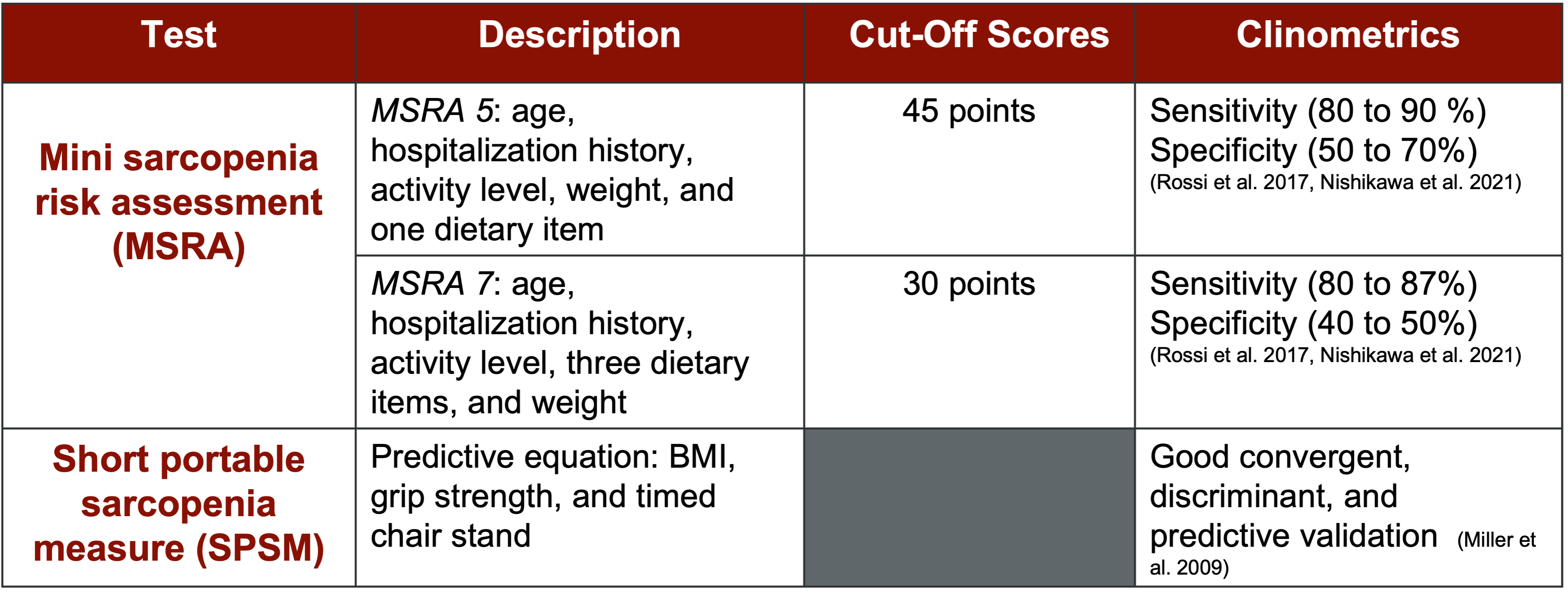
Figure 12. Other screening tests. (Click here to enlarge this image.)
Moving forward, exploring these screening tools can aid in early detection and intervention for sarcopenia, contributing to more effective programming and management strategies.
Clinical Measures
When working with clients diagnosed with sarcopenia, it's important to consider various clinical measures beyond just muscle strength. Figure 13 lists several of these measures along with descriptions of each.

Figure 13. Clinical measures for working with clients diagnosed with sarcopenia. (Click here to enlarge this image.)
Posture, range of motion, joint mobility, and gait function are all areas that require attention. Let's not overlook the significance of a breathing assessment; after all, the diaphragm is a muscle too, and its function can impact overall well-being. Additionally, balance and function assessments are crucial. We often assess the three balance systems—vision, vestibular, and somatosensory—but it's essential to recognize that individuals with sarcopenia may struggle with balance due to poor muscle co-contraction for stabilization. Therefore, integrating balance and function exercises into resistance training regimens is imperative. Now, let's delve further into this topic.
Bottom Line
In summary, it's evident that various screening tools and assessments are available, with the European method emerging as the most widely accepted for sarcopenia screening. Moreover, before designing resistance exercise and high-intensity interval training (HIIT) programs, conducting a comprehensive assessment is crucial.
The key takeaway here is that regardless of the assessment method used, the focus remains on evaluating muscle strength and quality. Ultimately, all indicators emphasize the importance of incorporating resistance training into intervention strategies.
Things to think about:
- Professionals should have an understanding of the different sarcopenia screening and assessments.
- The EWGSOP2 Algorithm seems to be the most widely accepted screening strategy.
- A comprehensive examination is needed prior to resistance exercise or HIIT exercise programming. (Kim & Kim 2023)
Module III: Fitness Assessment and Monitoring
In Module Three, we must tailor our approach based on the client's function and fitness level. Utilizing a different questionnaire may benefit higher-functioning clients or groups of seniors experiencing age-related muscle loss and balance issues.
The PAR-Q+ is a pre-participation screening tool comprising seven primary questions concerning an individual's health status. Often termed a readiness questionnaire, the PAR-Q+ is extensively used in research and fitness settings to assess potential health-related barriers to exercise. If a respondent answers yes to the initial seven questions, further exploration through a comprehensive four-page questionnaire is warranted. This should be updated by the patient every 12 months.
Originating from Canada, the PAR-Q offers a validated means to identify health concerns that may impact exercise participation. Integrating this form into the screening process can provide valuable insights, whether in a group setting or a personal training environment. Even post-physical therapy, having clients complete the PAR-Q before commencing a fitness regimen is advisable. For those working in cardiac rehabilitation or with lower-functioning individuals, the ePAR-med-X+ offers a similarly comprehensive approach online.
When it comes to fitness assessments, our approach remains comprehensive. We start with a health risk appraisal, ensuring we cover all bases. Additionally, integrating tools like the PAR-Q is crucial, especially for older individuals or those potentially affected by post-COVID-19 issues. Many clients may have experienced a lapse in regular medical check-ups during the pandemic, making it essential to inquire about recent assessments and blood tests.
In my practice, I always prioritize physiological assessments. I routinely measure resting blood pressure and pulse rate during the initial session. It's a simple yet essential step, providing a baseline for monitoring any changes as we progress with exercise prescriptions. This practice might not be universal, but I believe it's a necessary precaution.
Furthermore, assessing cardiorespiratory function, dynamic movements, muscle length, and performance through field tests offers valuable insights into a client's overall fitness status. While time and resources may limit the extent of these assessments, they can be incredibly beneficial if feasible. In my concierge practice, I often conduct these tests to track progress and ensure continuity of care, particularly when transitioning clients from physical therapy to fitness training. Sharing detailed reports with fitness trainers further enhances collaboration and client management. So, leveraging these assessments strategically can optimize client outcomes and streamline the transition between the healthcare and fitness realms.
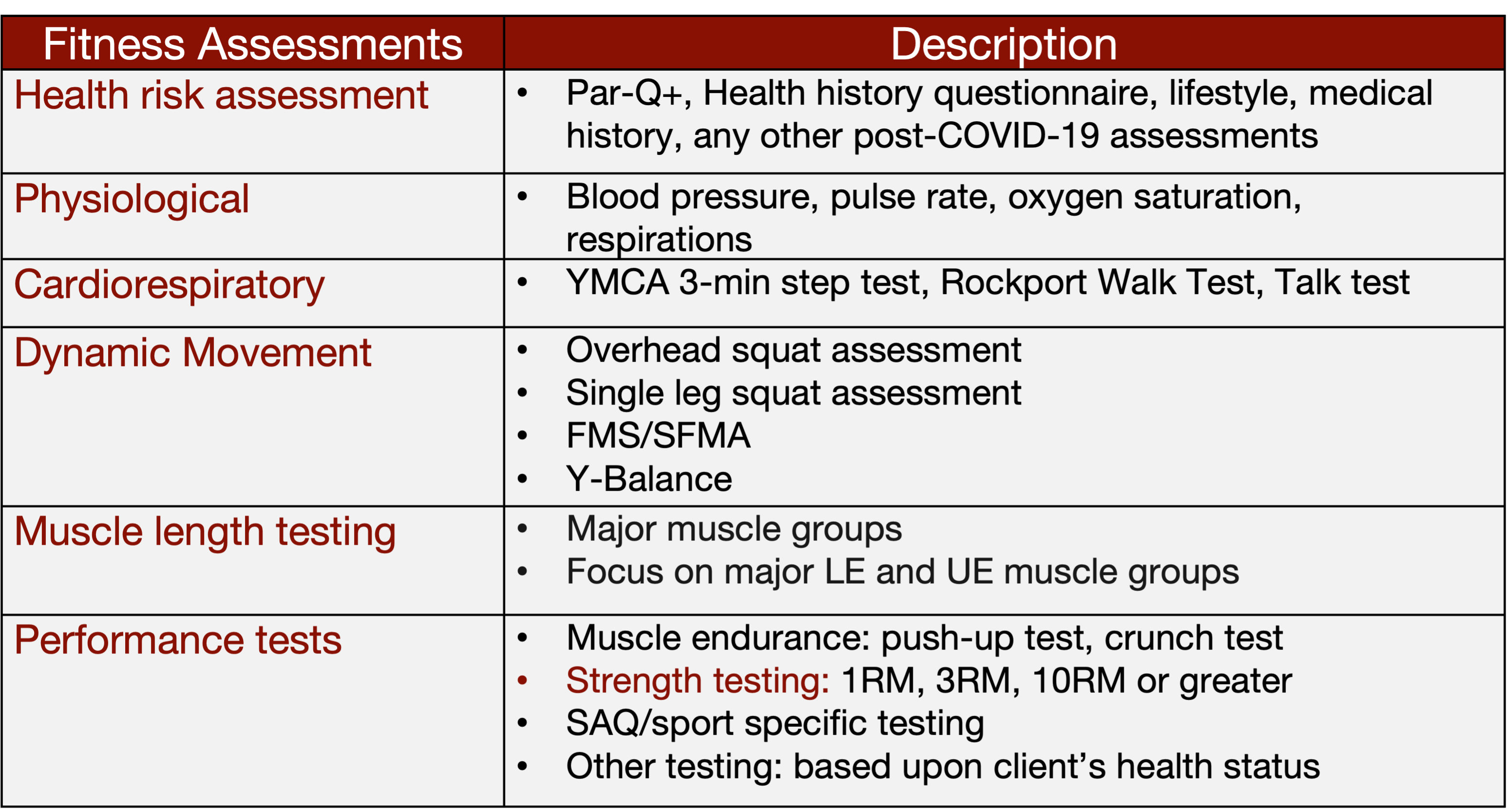
Figure 14. Fitness assessments. (Click here to enlarge this image.)
Biometric Tracking
Another valuable tool to consider for fitness tracking is heart rate variability (HRV). HRV measures the variation in time intervals between consecutive heartbeats, offering insights into the functioning of the autonomic nervous system throughout the day. Devices like the Whoop band and smartwatches now incorporate HRV monitoring, making it accessible for many individuals.

Figure 15. Biometrics to be tracked. (Click here to enlarge this image.)
Heart rate variability (HRV) is a relatively recent addition to the realm of biometric metrics despite having been studied since the early 2000s. It offers valuable insights into the regulation of heart rate by the autonomic nervous system over the course of the day. HRV reflects the dynamic interplay between the sympathetic and parasympathetic branches of the autonomic nervous system.
A crucial aspect of HRV analysis is the variability of heart rate patterns. A higher degree of variability, akin to the undulating peaks and troughs of a PQRST wave, signifies a harmonious collaboration between the sympathetic and parasympathetic systems. This variability is regarded as indicative of robust cardiovascular health. Conversely, a more consistent heart rate may suggest heightened sympathetic activity, potentially linked to factors such as pain, sleep disturbances, or other stressors affecting the body.
The integration of HRV monitoring extends beyond the realms of fitness and into healthcare. Its utility has been demonstrated in diverse applications, including the diagnosis of conditions like COVID-19. By scrutinizing subtle deviations in heart rate dynamics, HRV analysis holds promise as a diagnostic tool and a means of assessing how individuals manage various stressors.
While HRV monitoring presents exciting possibilities, it's essential to consider practical factors such as cost and patient demographics. Biometric trackers equipped with HRV capabilities can be expensive, prompting a thoughtful evaluation of their suitability for clinical use based on the patient population and available resources.
Patient Monitoring
Let's dive into a more straightforward approach with subjective assessments, specifically the Rate of Perceived Exertion (RPE) scale seen in Figure 16. You might be familiar with the original scale, which ranged from six to 20, but there's a newer version called the Borg Categorical Scale, or CR-10, which has been fully validated. Unlike its predecessor, the CR-10 employs the familiar zero to 10 scale, making it more intuitive for patients accustomed to Likert-style scales.
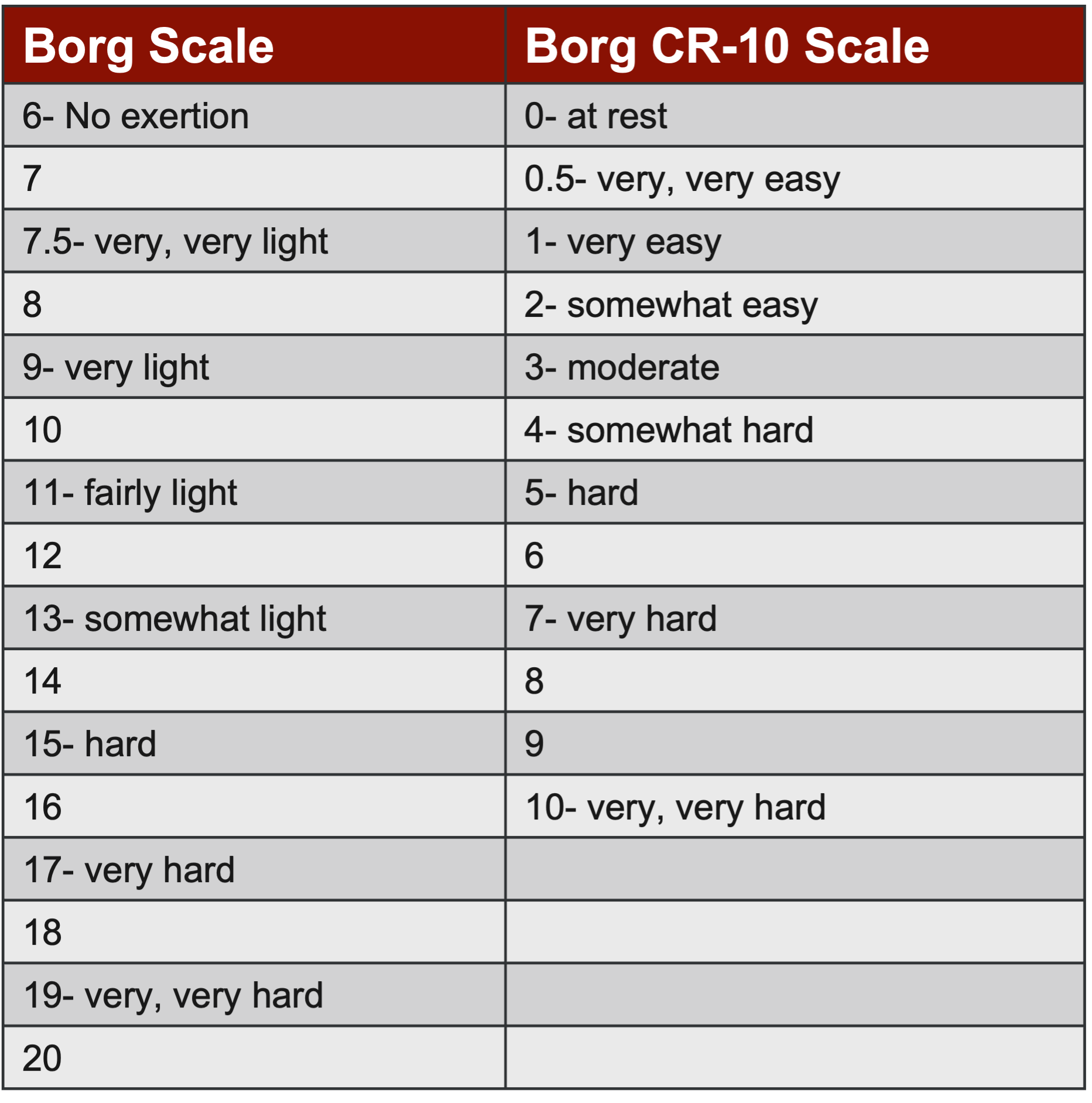
Figure 16. Rate of perceived exertion (RPE) scale (Helms et al. 2017, Lea et al. 2022).
During resistance training sessions, instead of determining a baseline with a one-, three-, or 10-rep max, many sports performance and strength and conditioning professionals are turning to RPE to guide weight selection. Here's how it works: Let's say you establish that Sherry can comfortably handle 15-pound dumbbells for 10 reps. You might start her off with that weight and then ask her to rate her perceived exertion on the RPE scale after completing a set. If Sherry reports a low RPE, indicating the weight was relatively easy, you can incrementally increase the load for subsequent sets.
Originally developed for aerobic exercise, RPE has now been adapted and validated for resistance training. By targeting a specific RPE, such as five, you can ensure that clients are exerting themselves appropriately without risking overexertion. For example, if John's RPE exceeds the target, you might adjust the weight or repetitions to keep the intensity within the desired range.
RPE is gaining traction in resistance and HIIT training because it offers a comfortable and effective means of gauging effort levels. Additionally, researchers in strength and conditioning advocate for loading the neuromuscular system with at least 65% of the one repetition maximum (1RM) to stimulate growth. Given its familiarity and utility, RPE is emerging as a preferred metric, particularly for older clients who may find it more accessible than traditional assessments. So, as you jot down notes, consider integrating RPE into your training protocols for a more personalized and effective approach.
Finally, let's address a critical consideration: avoiding overtraining in individuals with sarcopenia. It's crucial to remember that these individuals may have lost type two muscle fibers, leading to reduced strength and speed. Additionally, their respiratory capacity might be compromised. While their tidal volume may be within normal range, they could lack sufficient inspiratory muscle strength. This can manifest during activities like HIIT training, where rapid, intense breathing is required, potentially causing them to fatigue more quickly. So, as you design their exercise regimen, keep a close eye on their exertion levels and adjust the intensity accordingly to ensure optimal progress without risking burnout or injury.
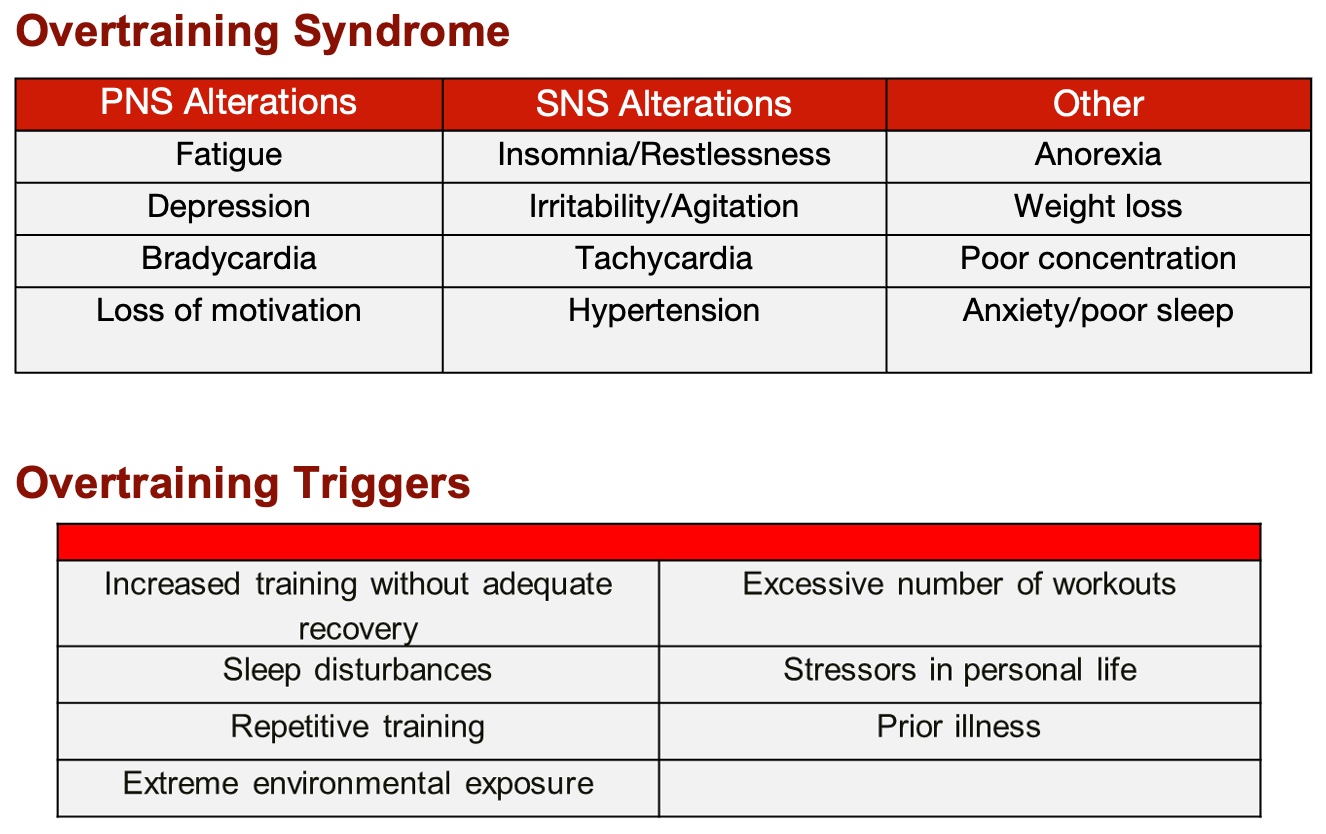
Figure 17. Overtraining syndrome and overtraining triggers. (Click here to enlarge this image.)
It's essential to recognize the signs of overtraining, especially in individuals with sarcopenia, as they can easily become fatigued and tired. Therefore, adopting a cautious approach is crucial, starting with linear periodization characterized by gradual and manageable progression. Additionally, remain vigilant for overtraining triggers, which encompass physical strain and psychosocial factors. By prioritizing a gradual and balanced training regimen while being mindful of potential stressors, we can mitigate the risk of overtraining and support the well-being of our clients with sarcopenia.
Bottom Line
In summary, integrating fitness-related assessments into your practice could be highly beneficial, particularly when working with aging individuals. Proper screening procedures are essential to ensure their safety and effectiveness in resistance or HIIT training programs. Utilizing biometric trackers, RPE scales, and other monitoring tools can aid in tracking progress and adjusting interventions as needed. The widespread adoption of RPE scales underscores their usefulness for both aerobic and anaerobic exercises, reflecting a growing body of research supporting their efficacy. Therefore, incorporating these strategies into practice can enhance the overall quality and effectiveness of interventions for individuals with sarcopenia and similar conditions.
Things to think about:
- Professionals should provide a fitness assessment prior to patients participating in a resistance exercise and/or HIIT.
- Patients with sarcopenia need to be monitored during exercise to ensure their safety.
- Biometric tracking via wearable devices is an emerging technology that allows for easy patient monitoring.
- Professionals should consider using the RPE scale to monitor the patients.
Module IV: Resistance Exercise Strategies
So, to recap, understanding the content covered in modules one and three is crucial for justifying the importance of resistance training and promoting longevity. Sarcopenia is no longer viewed as a simple diagnosis but rather as a multifaceted process influenced by factors such as dietary trends and medications. Recent advancements, like GLP-1 medications, are proving to be game-changers in the field. However, their cosmetic applications are overshadowing their potential benefits for conditions like type two diabetes, for which they were originally developed.
The overarching theme emphasizes the need to prevent sarcopenia through resistance and HIIT training. Now, let's delve into the concepts of resistance training tailored specifically for individuals with primary or secondary sarcopenia. Across the board, research consistently points to resistance exercise (RE) as the primary treatment modality. Physicians increasingly prescribe exercise programs as the initial step in managing sarcopenia, particularly following surgeries or prolonged periods of immobility. More medical professionals are recognizing its significance and integrating exercise prescriptions into patient care plans with the advent of specific diagnostic codes for sarcopenia.
Conversations surrounding resistance exercise (RE) programs are gaining traction, especially with influential figures like Peter Attia and medical professionals emphasizing the link between longevity and muscle strength. However, it's essential to recognize that RE isn't routinely prescribed yet. Nonetheless, there's a growing understanding that for RE to be effective, it must impose sufficient demands on the system, following the principle of Specific Adaptations to Impose Demands (SAID Principle). Without adequate loading, the system won't respond optimally.
In teaching exercise physiology at the university level, I stress the importance of understanding that the body requires time to adapt to exercise stimuli. Neurological return typically takes about four weeks, during which motor points and the primary motor cortex become more responsive. Following this, around six weeks of continuous training are needed for the neuroendocrine system to release growth factors and initiate hypertrophy. While these timelines may vary, especially in perioperative or post-surgical contexts, they provide a general framework for understanding physiological adaptations to exercise.
The Overload Principle, which suggests that exercise intensity should be around 65% to 75% of maximum capacity or higher, underpins effective resistance training (Yasuda, 2022). However, for individuals who may struggle with lifting heavier loads, methods like Rate of Perceived Exertion (RPE) offer an alternative means of gauging exercise intensity and progression. Ultimately, the goal is to design progressive exercise routines tailored to each individual's capabilities and goals, ensuring that even sedentary individuals can benefit from structured exercise programs.
Resistance Exercises and Sarcopenia
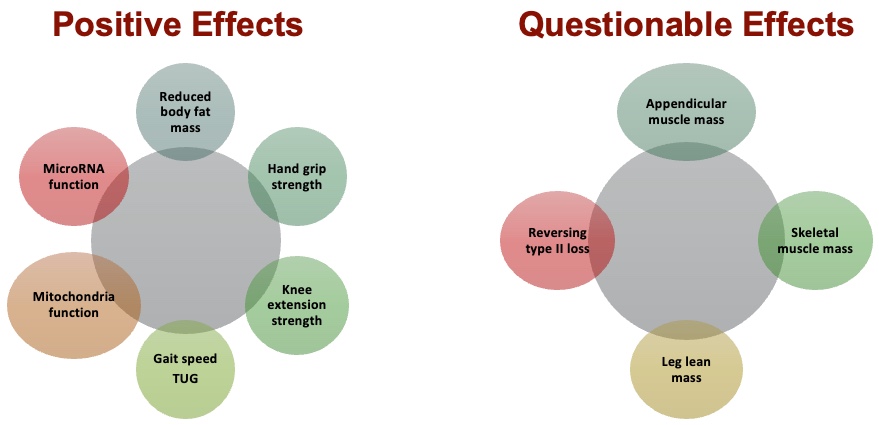
Figure 18. Effects of resistance exercises on those with sarcopenia. (Click here to enlarge this image.)
Research on resistance exercise (RE) and its effects on sarcopenia reveals a plethora of positive effects. These include reductions in body fat, increased strength in various muscle groups such as the hands and knees, improvements in gait and Timed Up and Go (TUG) scores, and notably, enhancements in mitochondrial function. Given the pathophysiological implications of sarcopenia, where mitochondrial function tends to decline with age, the observed improvements with continuous weightlifting are particularly significant. Furthermore, there's evidence suggesting that RE may stimulate increased microRNA function.
However, some effects of RE on sarcopenia remain subject to ongoing research. For instance, the extent to which RE contributes to the increase in appendicular muscle mass, skeletal muscle mass, leg lean mass, and the reversal of type two muscle fibers is still being investigated. While some studies affirm these effects, others are more cautious, acknowledging that while individuals may experience strength gains, significant muscle hypertrophy in older adults remains uncertain. Therefore, it's crucial to remain mindful of these areas that warrant further exploration.

Figure 19. Resistance exercises programming for sarcopenia. (Click here to enlarge this image.)
When it comes to programming for sarcopenia, it's important to note that this area is still relatively understudied. However, recent studies, such as those by Hurst et al. in 2022, offer some valuable insights. They suggest a minimum of two resistance training sessions per week, with loading intensities ranging from 40% to 60% or 70% to 85% of one's maximum, or an RPE range of three to five or six to eight, utilizing standard moderate-level variables.
Their recommendations align closely with the principles typically applied in phases one to three of post-rehabilitation programs, emphasizing one to three sets of six to 12 repetitions, with rest periods ranging from 60 seconds to two minutes. This framework allows for a gradual, manageable progression for clients, making it suitable for incorporating into home exercise programs.
Integrating full-body exercises early on, as part of rehabilitation protocols, can aid in maintaining overall conditioning and potentially expedite recovery. Hurst's guidelines offer a reasonable and evidence-based approach to programming for sarcopenia, providing a valuable reference point for practitioners seeking effective strategies in this area of treatment and management.
Chen's approach leans towards a slightly more aggressive stance, advocating for loading the system three days a week with higher reps and sets. On the other hand, Law's recommendations, introduced in 2016, strike a midpoint between these approaches. They typically involve three sets of 10 repetitions, a regimen commonly employed in physical therapy, with rest intervals of one to two minutes to facilitate ATP replenishment in the agonistic muscles.
This trend towards a middle ground is becoming more apparent in programming guidelines. However, if you're seeking a balanced and evidence-based approach, the Hurst reference stands out as a reliable choice. Its recommendations offer a comprehensive framework that balances effectiveness with feasibility, making it a valuable resource for practitioners navigating the complexities of sarcopenia management and treatment.
Types of Resistance Exercises for Sarcopenia

Figure 20. Types of resistance exercises for sarcopenia. (Click here to enlarge this image.)
When it comes to resistance training programming, researchers like Yasuda have provided valuable insights into different approaches. For younger and more capable clients, higher-load programming can be effective, ranging from 70% to 85% of their maximum capacity, performed two to three days a week with eight to 12 repetitions.
Another promising avenue is blood flow restriction (BFR) training, although its efficacy specifically for sarcopenia hasn't been thoroughly studied yet. Researchers are exploring the potential benefits of low-load BFR for muscle building in sarcopenic individuals, prompting the development of guidelines in this area.
Additionally, low-load, slow-movement exercises that focus on eccentric and concentric muscle actions and fatigue protocols offer alternative programming options. However, it's essential to exercise caution and consider individual suitability for these methods.
While some practitioners may incorporate BFR into their programming, especially for clients who can tolerate it, others may prefer to stick to more traditional approaches. As always, it's crucial to prioritize safety and effectiveness based on each client's unique needs and capabilities.
Furthermore, don't overlook the importance of progressions and regressions when designing resistance training programs. These tools, commonly used in fitness settings, allow for tailored adjustments to suit individual abilities and goals.
Resistance Exercises Progression/Regression
- Type of Exercise: Simple to Complex, Single Joint to Multi-Joint
- Surface: Stable to Unstable
- Environment: Known to Unknown, Anticipated to Unanticipated
- Exercise Tempo: Slow to Fast
- Base of Support: Double Leg to Single Leg
- Balance Systems: Eyes Open to Eyes Closed to Head Turns
- Planes of Motion: Sagittal to Frontal to Multi-plane
- Load: Light to Moderate to Heavy
When it comes to progressions in resistance training, the key is to gradually advance from simpler to more complex movements, from single-joint to multi-joint exercises, and from stable to unstable environments. Consider the environment in which you're training, the tempo of the exercises, the base of support, and the integration of balance systems. Additionally, pay attention to the range of motion you're targeting and the load you're using.
Understanding that progressions and regressions in exercise programming send specific messages to the central nervous system is important. These messages dictate how the muscles will respond to the training stimulus. Therefore, it's essential to be deliberate in your choice of progressions and regressions, considering the desired outcome and the individual's goals and functional status.
Remember, the central nervous system receives, processes, and responds to these messages. The desired response should align with the individual's needs and objectives, as determined by thorough assessment and goal setting. So, when planning resistance training programs for individuals with sarcopenia, carefully consider the messages you're sending to their central nervous system to optimize training outcomes.
Resistance Exercise Training Tools

Figure 21. Resistance exercise training tools. (Click here to enlarge this image.)
It's important to consider various resistance training tools and methods when designing programs for clients, especially those with sarcopenia. Traditional tools like dumbbells, kettlebells, and machines offer constant resistance, while alternative tools like bands, suspension trainers, sandbags, and battle ropes provide variable resistance and can add diversity to workouts.
Using alternative tools not only adds variety but can also make workouts more enjoyable for clients. For example, suspension training (TRX) can be particularly effective and safe when done correctly, even for older individuals or those with mobility limitations. Additionally, sandbags offer instability while maintaining consistent mass, providing unique challenges for the muscles.
Furthermore, emerging techniques like blood flow restriction (BFR) training show promise in improving muscle strength and mass, particularly in older individuals. While BFR requires careful application and monitoring, it may be worth exploring for clients with sarcopenia under appropriate supervision. Ultimately, incorporating various resistance training tools and methods allows for personalized and effective programming tailored to the individual needs and capabilities of clients with sarcopenia.
I have a real passion for suspension training, and I'd like to share some exciting ideas with you. I've found it to be incredibly beneficial, not only for older individuals but also for clients recovering from strokes. When done safely, it can yield excellent results. Additionally, other tools like battle ropes, resistance bands, and stability balls can add an element of enjoyment to workouts. The best part is that many of these alternative tools require minimal space, making them perfect for clinics with limited room.
Consider incorporating these alternative tools into your sessions if you tend to stick to more traditional methods. For instance, sandbags are a fantastic option because they introduce instability while maintaining consistent mass. This instability challenges muscles in a unique way, promoting overall strength and balance.
Furthermore, emerging techniques such as blood flow restriction (BFR) training show great promise, especially for building muscle size and strength in older individuals. While implementing BFR requires careful consideration and monitoring, it can significantly enhance the effectiveness of resistance training programs. BFR has not been studied in older adults with sarcopenia, but Baker et al. (2020) and Cahalin et al. (2022) are pushing research to be conducted on older individuals.
Don't overlook the potential of alternative tools and techniques when designing resistance training programs. These options can not only diversify workouts but also contribute to improved strength, balance, and overall fitness levels.
Resistance Exercise Mixed Training Models
As we transition from conventional weight training to a more diverse approach, it's crucial to explore the effectiveness of mixed training. This integrative approach combines various alternative methods alongside traditional resistance training. For instance, imagine someone seated on a physio ball for balance while performing TRX exercises or engaging in one-legged RDLs with a sandbag. Mixed training has gained traction in sarcopenia research due to its adaptability and accessibility, especially in settings where traditional equipment may be limited.
In mixed training, practitioners utilize a variety of tools, such as weighted sandbags, resistance bands, free weights, and even machines, to create a multimodal training regimen. The underlying principle remains consistent: as long as individuals are lifting a resistance object, they're engaging in resistance exercise and challenging their musculoskeletal system. This approach opens up possibilities beyond traditional weightlifting, allowing for circuit training and high-intensity interval training (HIIT), which essentially is high-level circuit training.
In mixed training, researchers have introduced the FITTE-VP model, encompassing several key components. First, there's frequency, which typically ranges from two to five days per week, with an emphasis on moderate to high-intensity loads, ideally exceeding 65% of one's capacity. Sessions typically last between 30 to 60 minutes, focusing on bodyweight resistance exercises that promote enjoyment while ensuring adequate volume and progression over time.
FITTE-VP
- Frequency: 2-5 training days per week
- Intensity (Load): Moderate to high intensity exercise
- RM: ~ 65% progress to ≥ 85% 1 RM
- Reps: 6 to 15, Sets: 1 to 4
- Time: 30-60 minutes
- Type: BW, resistance bands, free-weights, machines, & alternative tools.
- Enjoyment: Optimal environment
- Volume: TBD
- Progression: Use %1RM, Repetition continuum, or RPE
The underlying principle of mixed training aligns with the integrated neuromuscular concept, emphasizing proprioceptive-rich environments. This may involve exercises performed on unstable surfaces like a BOSU ball, challenging individuals to engage their neuromuscular system more comprehensively.
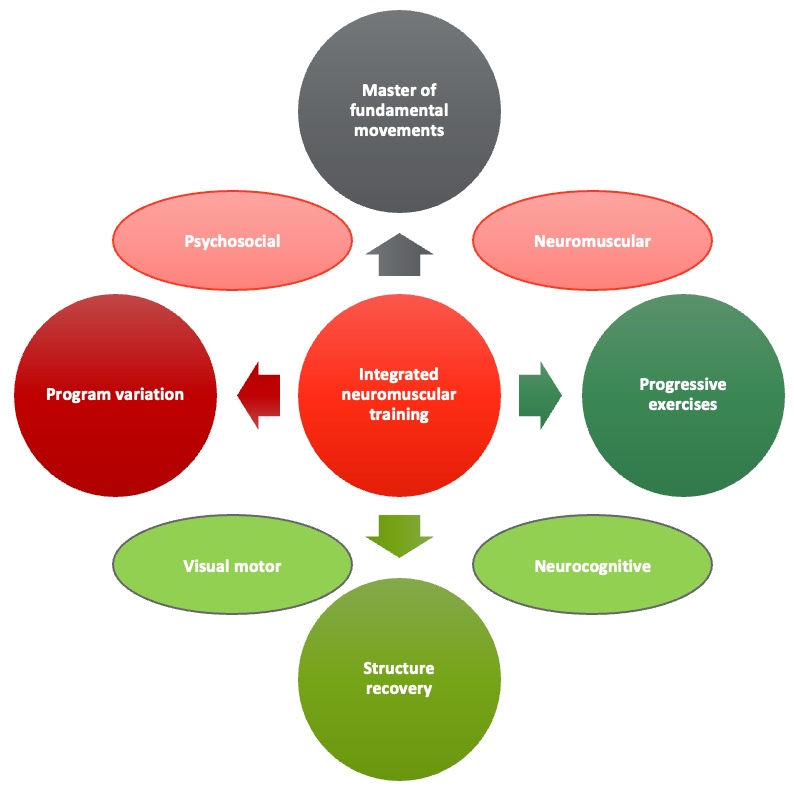
Figure 22. Integrated neuromuscular training (Meyer, et al. 2016). (Click here to enlarge this image.)
Many researchers are advancing beyond traditional weightlifting approaches for sarcopenia, advocating for holistic body challenges and potentially better outcomes. One notable model is the National Academy of Sports Medicine's five-phase linear progressive model, which I've had experience with in the past. It's structured to first stabilize the body, then focus on endurance, muscle building, strength, and ultimately, power development.
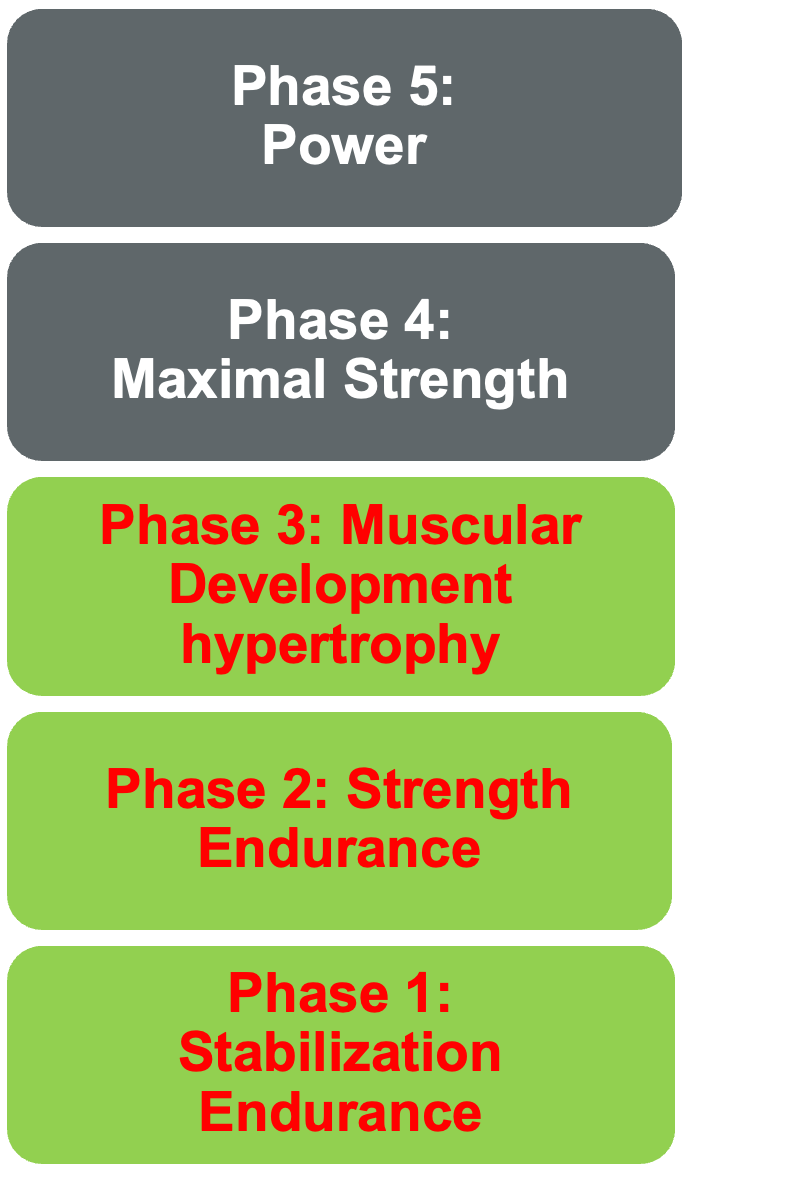
Figure 23. The National Academy of Sports Medicine's Optimum Performance Training (OPT) Model.
This linear progression model is widely used in sports performance realms and sports physical therapy. It emphasizes fundamental movements like pushing, pulling, squatting, and hinging for stabilization, followed by proprioceptive challenges to strengthen the core and improve balance. As we move through phases, the focus shifts to endurance, muscular development, and strength building.
This model offers a comprehensive approach for those considering implementing a fitness program in their clinical practice to address sarcopenia. It's well-explained in online resources and has been developed by an interprofessional team, including PTs, OTs, medical doctors, and chiropractors. I've personally found it effective in teaching settings from PhD students to undergraduates.
When it comes to resistance training best practices, while research on ideal programming is still limited, the Hurst program is a solid option for general resistance training. For mixed training, the FITTE-VP model provides a structured framework to follow. These two approaches offer practical guidelines for designing effective resistance training programs for individuals combating sarcopenia.
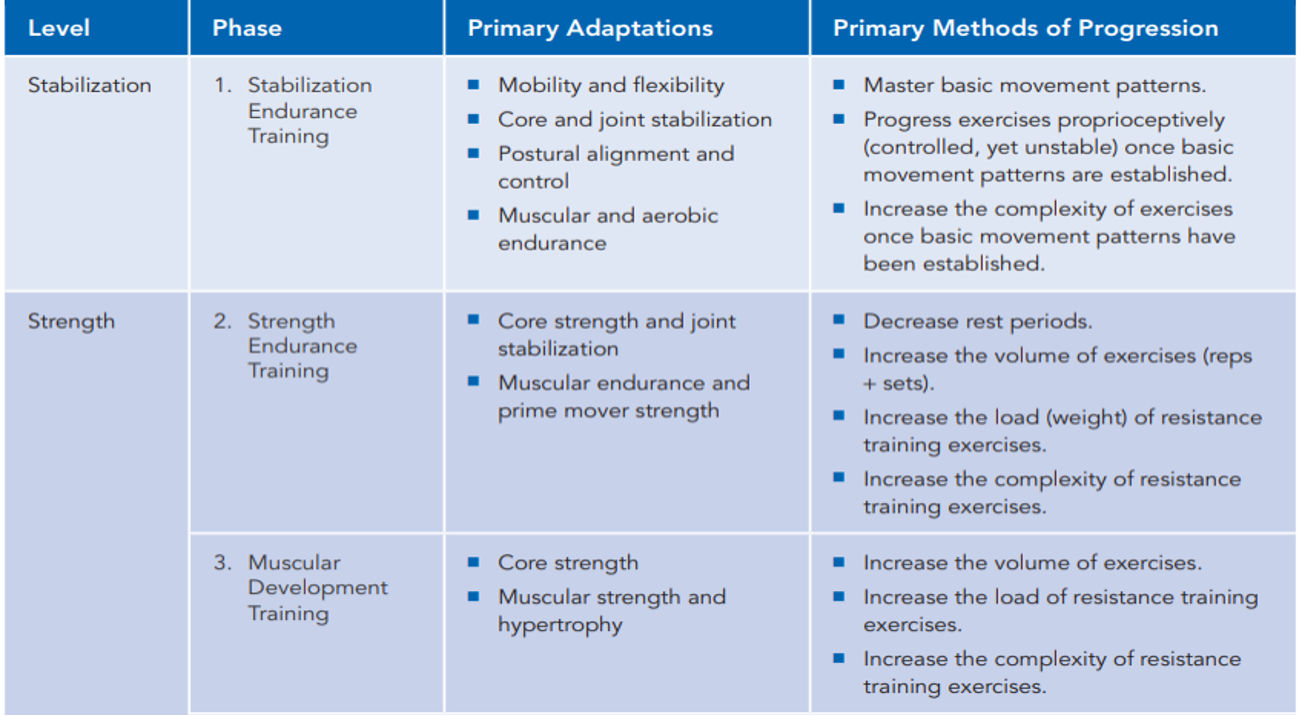
Figure 24. NASM OPT Model. (Click here to enlarge this image.)
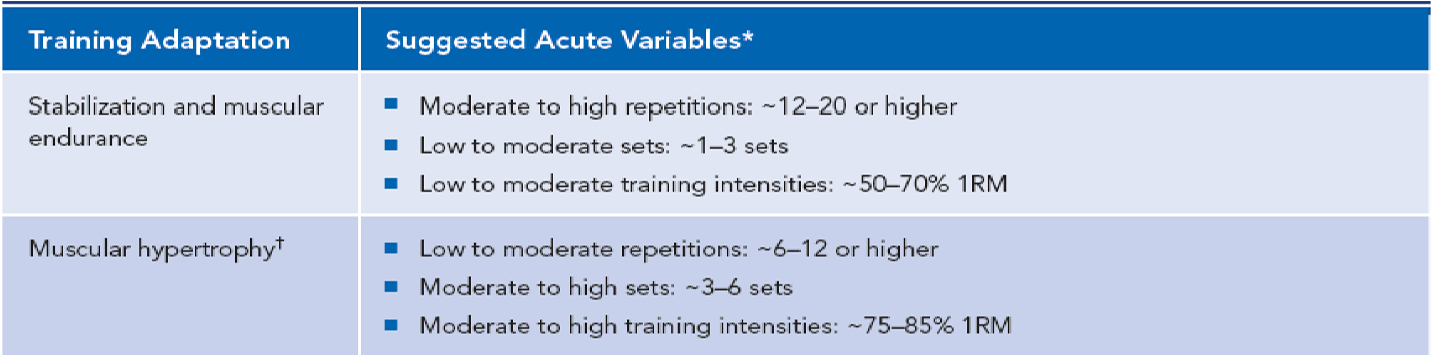
Figure 25. NASM OPT Model training adaptations. (Click here to enlarge this image.)
Resistance Exercise Best Practices
Regarding best practices, I advocate for an integrated approach to programming. I believe in incorporating resistance training, core work, balance exercises, speed and agility drills, plyometrics, and proper warm-up and cool-down routines. In many clinical settings, it's feasible to prescribe such a comprehensive regimen.
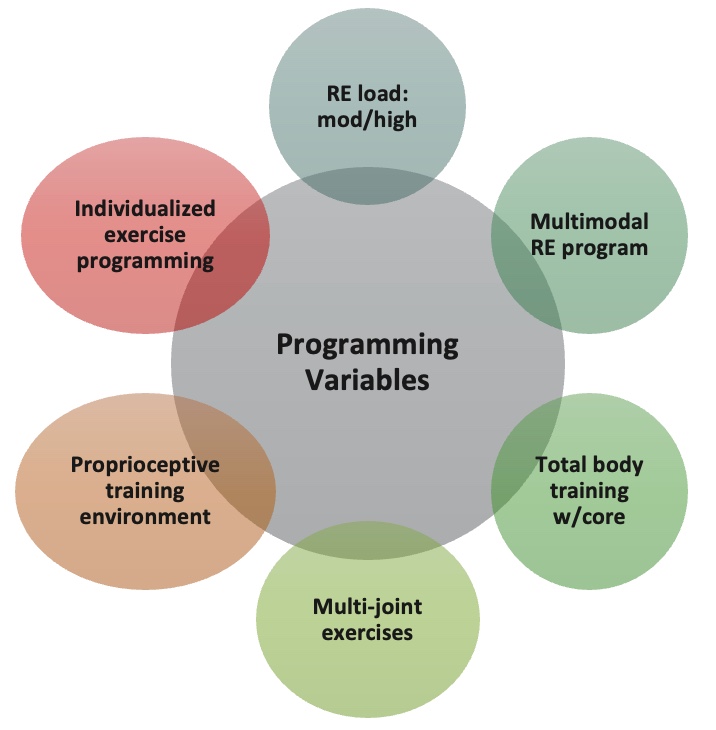
Figure 26. Programming variables for resistance exercises.
Programming variables offer ample opportunities to challenge the body. For instance, plyometric exercises like jump rope can be suitable for clients in their 50s or 60s who can handle it. Advanced movements such as plank rows or single-leg squats with a dumbbell press also integrate core work and resistance training effectively.
Integrated programming still prioritizes loading the system with resistance, whether through bodyweight exercises or external weights. It's essential to recognize that body weight itself serves as a form of resistance, underscoring the versatility of this approach.
In the realm of resistance training, the past five years have seen a surge in systematic reviews and studies highlighting its efficacy for sarcopenia. While the evidence overwhelmingly supports resistance training, there's still a lack of comprehensive exercise prescription guidelines. However, ongoing research efforts, including proposals for new studies, indicate that this area is gaining recognition.
Researchers like Hurst and Geng, whose work I've referenced from 2023, are contributing to the development of guidelines and recommendations. As we await further advancements in this field, it's crucial to consider traditional resistance training methods alongside mixed training approaches. Additionally, incorporating subjective measures like Rating of Perceived Exertion (RPE) or percentage of repetition maximum (rep max) can enhance the precision of exercise prescription. This update is a snapshot of the current evidence-based landscape in resistance training for sarcopenia.
Selected Reviews (past 5 years):
- 2023- Shen et al. J Cachexia Musc (+ RE)
- 2023- Zeng et al. Geriatric Nursing (+ RE)
- 2023- Long et al. Ageing Res Rev (+ RE)
- 2023- Geng et al. Medicine (+ RE/Mixed)
- 2022- Hurst et al. Age Aging (+ RE)
- 2022- Yasuda. Cells (+ RE)
- 2021- Chen et al. Eur Review Aging Phys Act (+ RE)
- 2021- Lu et al. BMC Geriatrics (+) (RE/Mixed)
- 2018- Vietstra et al. Australas J Ageing (+ RE)
Consensus:
- Moderate evidence for therapeutic benefits
- Improved muscle strength and performance
Bottom Line
Professionals widely acknowledge that integrating resistance training (RE) into programming is advisable, even though the research landscape is still evolving. Despite the limited evidence on practices like Blood Flow Restriction (BFR), recommendations for their use persist. While studies exist on resistance training and sarcopenia, definitive programming variables for optimal outcomes are yet to be established.
As clinicians, we seek practical solutions rather than wading through numerous studies. We're ultimately looking for the best recipe – the optimal approach. However, reaching that point will take time. In the meantime, we must rely on our clinical judgment, grounded in foundational principles such as loading the system to at least 65% of capacity, among others we've discussed.
Things to think about:
- Professionals should consider integrating RE into their patient programs.
- The research on RE for individuals with sarcopenia is still emerging. The available research supports RE as a 1st line intervention.
- Researchers suggest that RE must be ≥ 65-75% 1RM to stimulate a muscular response. This load may be limited in some patients.
- Different types of RE programming such as BFR and integrated training may further challenge the patient.
- Researchers suggest that neural mechanisms (e.g., rate coding) may be the primary reason for increased muscle strength in individuals with sarcopenia.
- Researchers suggest that neural mechanisms (e.g., rate coding) may be the primary reason for increased muscle strength in individuals with sarcopenia.
Module V: HIIT Strategies
Alright, let's dedicate our final moments to discussing HIIT training. It's crucial to note that HIIT training has seen a surge in research within the older population, particularly in the realm of sarcopenia. As you know, high-intensity interval training is just one element within a spectrum of diverse exercises.

Figure 27. Types of interval training. (Click here to enlarge this image.)
Let's begin by revisiting the original moderate-intensity continuous training, which was long considered the gold standard. It's akin to circuit training, moving from one station to the next or engaging in regular, sustained exercise. However, for our focus on sarcopenia, it's important to note that while cardio contributes to overall health, it's not the primary factor in addressing sarcopenia. Resistance training takes the spotlight here.
Several years back, the exploration of high-intensity interval training (HIIT) began. HIIT involves pushing through a circuit of various exercises at maximum effort—whether it's ball slams, jumping jacks, or explosive bodyweight movements like jump squats. The aim is to comprehensively tax the body's bioenergetic systems, including the PCR, glycolytic, aerobic, and nervous and musculoskeletal systems.
HIIT training yields both aerobic and anaerobic benefits. However, it's undeniably demanding on the body. Many individuals, particularly older adults, may find moderate-intensity continuous training (MICT) more appealing due to its lower intensity. Balancing the intensity level becomes crucial, especially considering potential complications such as osteoporosis.
Recent research suggests that older individuals can handle higher-intensity training despite its challenges. Studies published in reputable journals like the Journal of Strength and Conditioning Research indicate that individuals in their 70s and 80s can tolerate training at 75% to 85% intensity.
In addition to HIIT training, other forms of interval training include sprint interval training and repeated sprint training. However, HIIT remains the focus of current research on older populations, showing promise as an effective training modality for addressing sarcopenia.
High-Intensity Interval Training (HIIT)
- HIIT is characterized by repeated short to long bouts of high-intensity exercise followed by alternate recovery periods of low-intensity exercise or rest.
- Exercise intensity: ≥90%VO2max or >90–95% HRmax for 6 s to 4 min
- Recovery periods: low-intensity exercise or rest (20% to 40% VO2max for 10 s to 5 min)
- Low volume: ≤ 15 min work, High volume: ≥ 15 min work (Atakan et al. 2021)
High-intensity interval training (HIIT) for individuals with sarcopenia is characterized by repeated short to long bouts of high-intensity workouts. The general prescription typically involves exercising at intensities greater than 90% of VO2 max or 90% to 95% of heart rate max, with durations ranging from six seconds to four minutes.
Following these intense bouts, the recovery period can vary widely, spanning from 10 seconds to five minutes, depending on individual capacity and goals. As for the volume of HIIT training, it's often categorized as low volume if the total duration is less than 15 minutes, while anything longer is considered high volume.
Suggested HIIT Programming
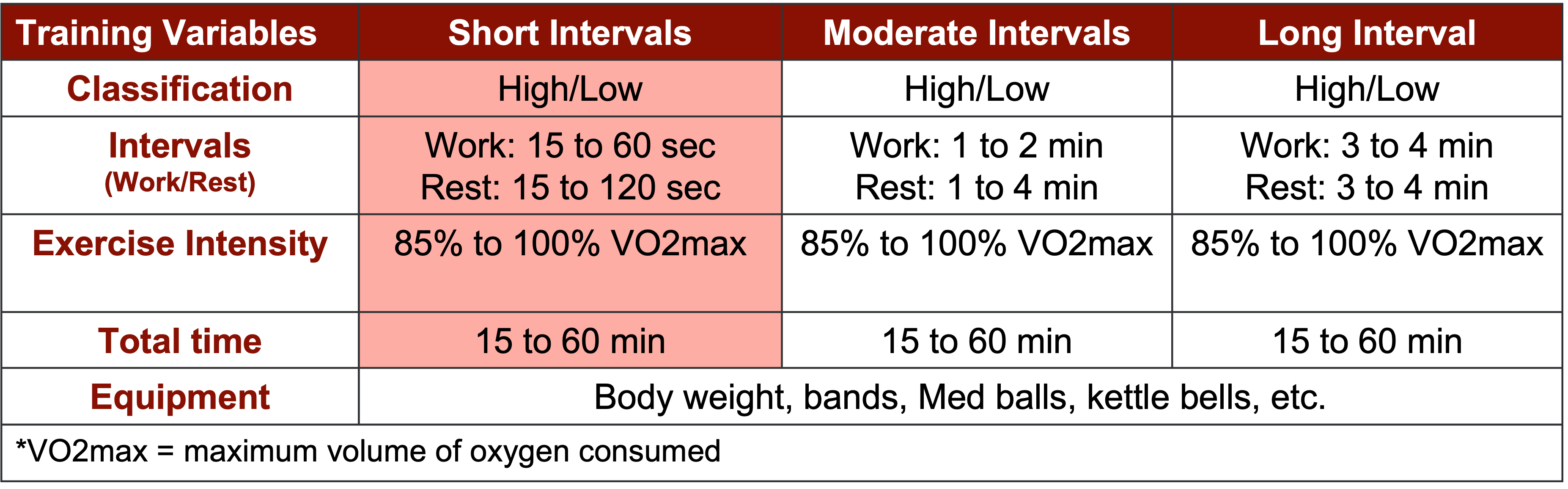
Figure 28. Suggested HIIT programming. (Click here to enlarge this image.)
HIIT training guidelines for individuals with sarcopenia are beginning to take shape, with researchers like Liu et al. providing valuable insights. One highlighted approach seen in Figure 28 involves utilizing work-to-rest ratios of one to one or one to two, which emphasizes alternating high-intensity intervals with lower-intensity recovery periods. This high-low pattern, particularly in short intervals, has gained popularity.
In practice, short bouts of intense exercise lasting around 15 seconds are followed by equally short rest periods before transitioning to the next exercise or station. This setup allows for dynamic movement sequences, either at stations or in place, depending on available space. For example, a patient might alternate between jumping jacks and planks, resting briefly between each exercise. This short-interval approach is often considered practical and feasible across various clinical settings.
Documented Benefits of HIIT
HIIT training has been extensively studied across diverse populations, ranging from young individuals to older adults, as well as recreational enthusiasts to professional athletes. Here are the documented benefits of HIIT.
- Improves mood, depression, and anxiety
- Decreases risk of breast cancer
- Decreases risk of cardiovascular disease
- Increases metabolic rate
- Lower fat mass
- Reduced incidents of osteoarthritis
- Reduces risk of metabolic syndrome
- Helps relieve low back pain
- Increases insulin sensitivity and lower risk of type 2 diabetes
- Lowers risk of colon cancer
- Reduces elderly falls
HIIT and Sarcopenia
HIIT training has garnered substantial attention in research across various demographics, including studies focusing on sarcopenia. These studies indicate significant benefits such as increased muscle mass, muscle quality, physical performance, muscle strength, muscle power, and improvements in mitochondrial function. The types of HIIT training explored in research encompass a range of approaches, including resistance training HIIT, aerobic sprint intervals, bodyweight exercises, mixed routines, and Tabata protocols. A blend of resistance and mixed HIIT training is commonly implemented in clinical settings. For instance, a session may involve exercises like squat presses with dumbbells, rapid monster walks with resistance bands, and push-ups on a BOSU ball. Through these findings, it's evident that both resistance training and HIIT training play crucial roles in mitigating or potentially slowing down the progression of sarcopenia.
HIIT Best Practices
When it comes to HIIT training, adhering to best practices is paramount. Establishing an appropriate work-to-rest interval and ratio is crucial to ensure effective recovery. Safety is of utmost importance, necessitating thorough client screening to mitigate any potential risks. Managing the number of repetitions, stations, circuits, and overall volume is essential to prevent overtaxing individuals. As a general guideline, limiting HIIT training sessions to two days per week is recommended to strike a balance between effectiveness and recovery.
Figure 29. HIIT programming variables (Buchheit & Laursen, 2013). (Click here to enlarge this image.)
HIIT Systematic/Literature Reviews
Selected Reviews (past 5 years):
- 2023 Zhu et al. Front Physiol (+)
- 2021- Hayes et al. Front Physiol (+) SR
- 2023- Kumar et al. Eur Geriat Med (+) SR
- 2021- Wu et al. Exp Gerontol (+) SR
- 2022- Liu et al. Clin Interv Aging (+)
- 2021- Atakan et al. Int J Environ Public Health (+)
Consensus:
- Moderate evidence for therapeutic benefits
- Improved muscle strength and performance
The systematic reviews have shown a noticeable uptick in literature since 2021, particularly concerning the surge of studies on HIIT training and its effects on individuals with sarcopenia post-pandemic. However, it's crucial to remember that HIIT training isn't suitable for everyone. As a clinician, assessing whether resistance training or modified HIIT training would be safer and more appropriate for each individual is essential. Personally, I typically initiate clients who are new to exercise or at a low level with resistance training before progressing to continuous moderate circuits and eventually integrating HIIT training into their regimen. Regardless of the approach chosen, resistance exercise remains the cornerstone in combating sarcopenia.
HIIT Example: Aerobic (Short-Intervals)
Let's explore some examples, especially for those not well-versed in HIIT training. Here's an aerobic circuit to cater to my fellow runners and aerobic enthusiasts. The circuit seen in Figure 30 entails a one-to-one work-to-rest ratio, encompassing five stations with high-intensity intervals denoted in green and rest periods in red. Participants engage in slow movement during the rest periods. You can complete the circuit twice or adjust as needed, targeting 10 to 30 minutes of exercise, focusing on the green segments. For instance, you could utilize the stationary bike, alternating between 30-second sprints and 30-second slow-paced intervals. This format is adaptable to various cardio machines like the elliptical or treadmill. While it's a viable option for some patients, remember that prioritizing weightlifting remains paramount.
Figure 30. Aerobic HIIT programming (short intervals).
HIIT Programming
- Intensity: 85 to 100%
- Work time: 30 sec
- Rest time: 30 sec (slow movement)
- Total exercise time: 10 to 30 min
- Warm-up/cool-down
- Running, elliptical, or stationary bike
HIIT Example: Bodyweight (Short-Intervals)
Regarding bodyweight HIIT training examples, we're targeting individuals in the stabilization phase or those with lower functionality. For beginners, exercises may include chair sit-to-stands, standing marches behind a chair, seated upper extremity movements, chair side lunges, and single-leg balances on the chair. These exercises typically operate at around 40% intensity due to the participants' lower capacity. Thus, it's crucial to establish robust work-to-rest ratios. For instance, participants might work for 15 seconds, followed by a rest period of 30 or 60 seconds. Given their limited training experience and aging status, starting slow is imperative, following the principles of linear periodization.
Figure 31. Bodyweight HIIT example (low functioning).
HIIT Programming
- Intensity: 40 to 85%
- Work time: 15 to 20 sec
- Rest time: 30 to 60 sec
- Total exercise time: 5 to 15 min
- Warm-up/cool-down
As individuals progress to the beginner stage of bodyweight training, exercises such as pushups, modified pushups, squats, sidestepping, forward lunges, and planks become suitable. Intensity increases by approximately 20% and adjustments in work-to-rest ratios may be necessary. Incorporating stations into the circuit requires accounting for transition time between exercises, typically around 15 seconds. Therefore, rest periods may vary, ranging from 30 to 45 seconds, to accommodate both exercise intensity and transition time.
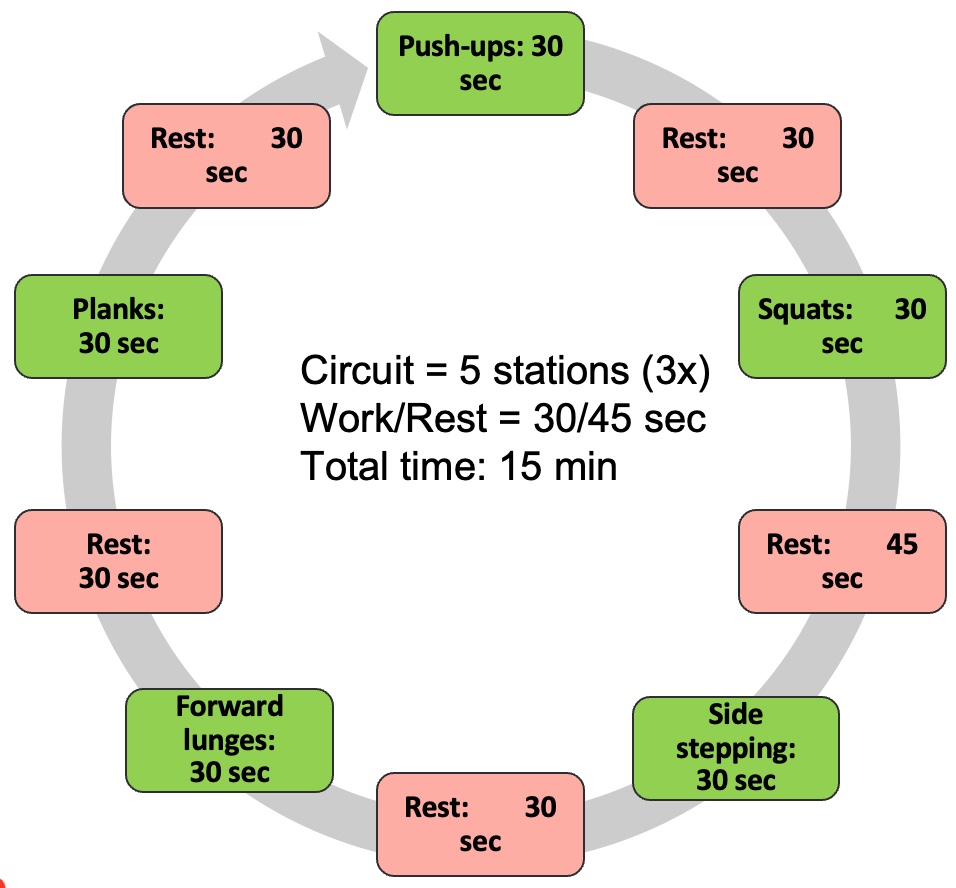
Figure 32. Bodyweight HIIT example (beginner).
In programming, particularly when utilizing stations, allocating around 15 seconds for participants to transition between exercises is essential. Hence, the rest periods indicated in the programming example—mostly 30 seconds—might occasionally be extended to 45 seconds to accommodate transition time. For instance, the sequence could involve intervals of 30 seconds of exercise followed by 30 seconds of rest, with an additional 15-second interval for transitioning between stations. Thus, it's crucial to consider this variability when designing the workout regimen.
HIIT Programming
- Intensity: 65% to 100%
- Work time: 30 sec
- Rest time: 45 sec
- Total exercise time: 15 min
- Warm-up/cool-down
For individuals at the intermediate level, perhaps showing early signs of sarcopenia but maintaining a high activity level, exercise intensity typically reaches around 75%. Assessing intensity using the Rating of Perceived Exertion (RPE) scale can be beneficial in this regard. Their workout regimen may include more dynamic movements like skipping, bounding, jump squats, forward lunges, jumping jacks, and planks with leg movements, reflecting a higher level of challenge. This level of activity might cater to the Weekend Warrior demographic if such a program is in place.
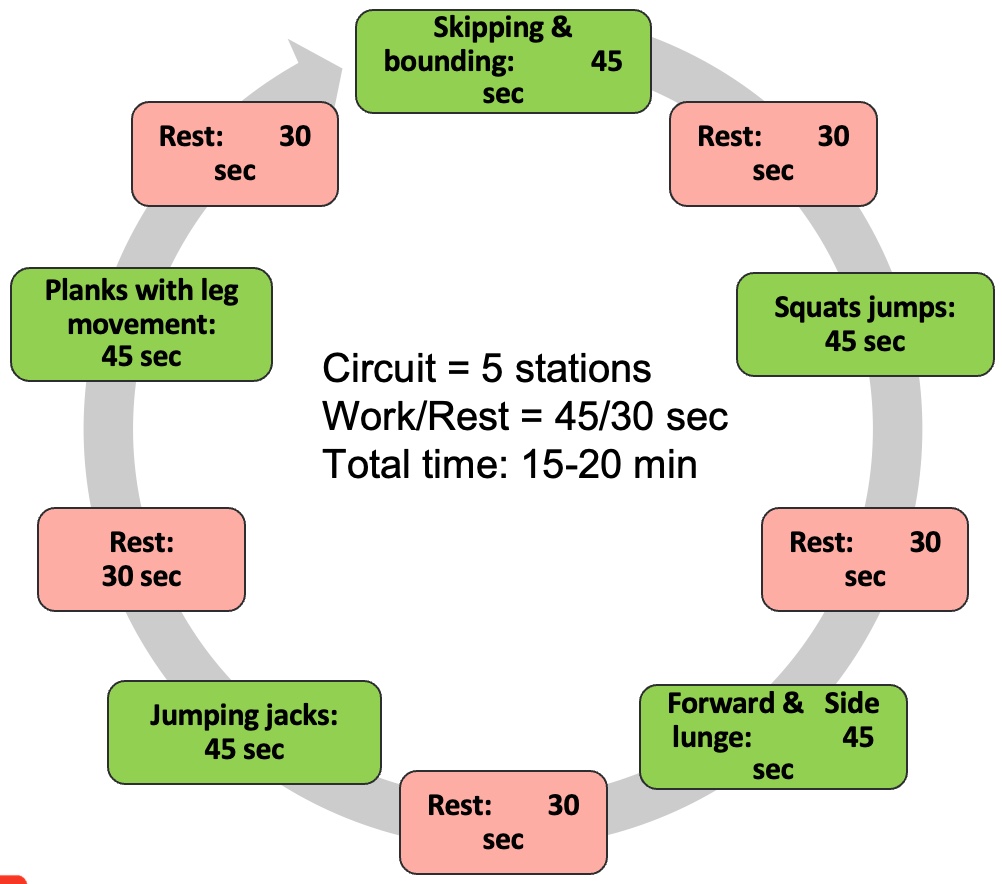
Figure 33. Bodyweight HIIT example (intermediate).
Regarding work-to-rest ratios, intervals like 40 seconds of exercise followed by 30 seconds of rest could be appropriate, given their better fitness level. However, it's essential to allow slightly more time for transitions between exercises due to the higher intensity. The key principle here is to ensure constant movement during rest periods. Participants should engage in active rest, such as walking around, rather than remaining sedentary.
HIIT Programming
- Intensity: 75% to 100%
- Work time: 45 sec
- Rest time: 30 sec
- Total exercise time: 15 to 20 min
- Warm-up/cool-down
For advanced participants, the focus shifts to exercises that challenge their musculoskeletal system and overall fitness to the fullest extent. Activities such as burpees, tuck jumps, skaters, jump rope, and agility drills become prominent, representing more traditional body weight HIIT training. Intensity levels typically exceed 85%, with stations lasting for 60 seconds or more, demanding considerable effort.
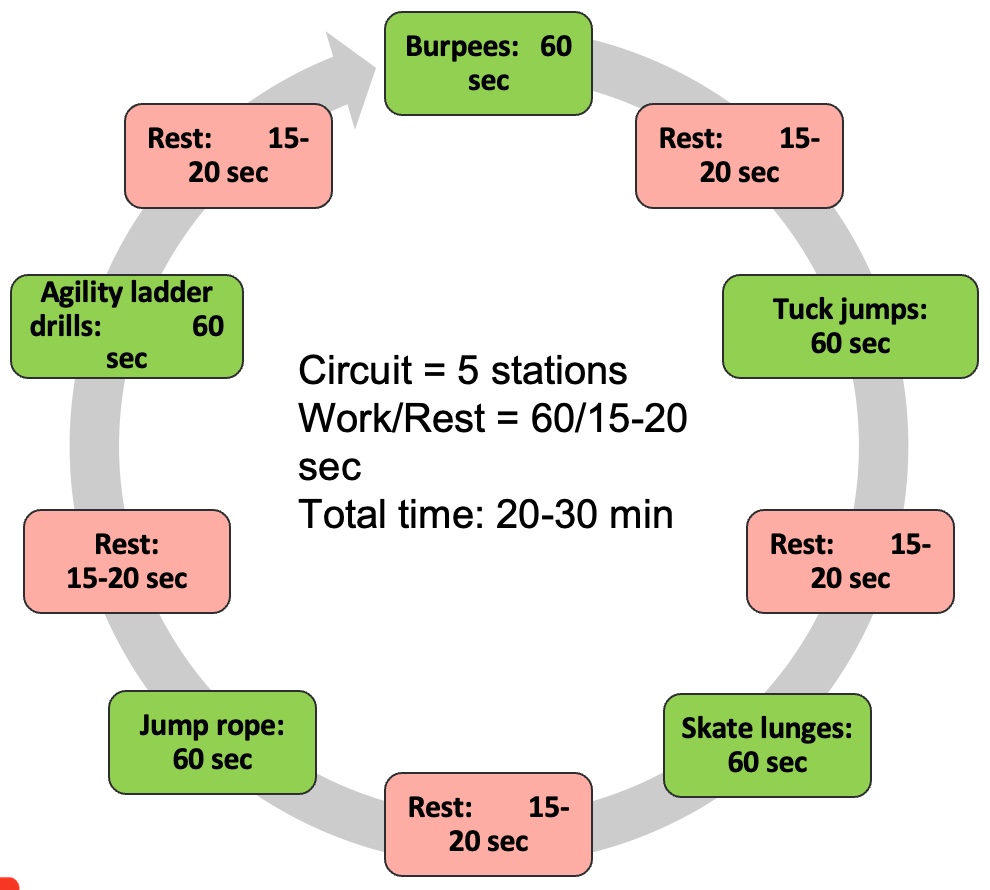
Figure 34. Bodyweight HIIT example (advanced).
Short rest periods are characteristic of advanced HIIT workouts, emphasizing continuous activity throughout the session. The goal is to maintain an active pace from start to finish, completing the routine within approximately 30 minutes, including warm-up and cool-down periods.
HIIT Programming
- Intensity: 85% to 100%
- Work time: 60 sec
- Rest time: 15 to 20 sec
- Total exercise time: 20 to 30 min
- Warm-up/cool-down
These examples illustrate the spectrum of body weight training, ranging from gentle beginnings to rigorous advanced routines, offering a comprehensive approach to fitness.
HIIT Example: Resistance Exercise (Short-Intervals)
For resistance training using short intervals, there are distinct exercises tailored to various proficiency levels. Remember, Figure 28 covered short, moderate, and long intervals. Short intervals are the most reasonable for the clinical setting.
For beginners, a cable standing lunge to a press, cable standing row to squat, step with a medicine ball hold, monster walks with a loop band, and goblet squats with a kettlebell form a comprehensive circuit. These exercises emphasize basic movements and are performed with an intensity of around 65%. Work-to-rest ratios typically include 30 seconds on and 30 seconds off, with an additional 15 seconds for transitioning between exercises.
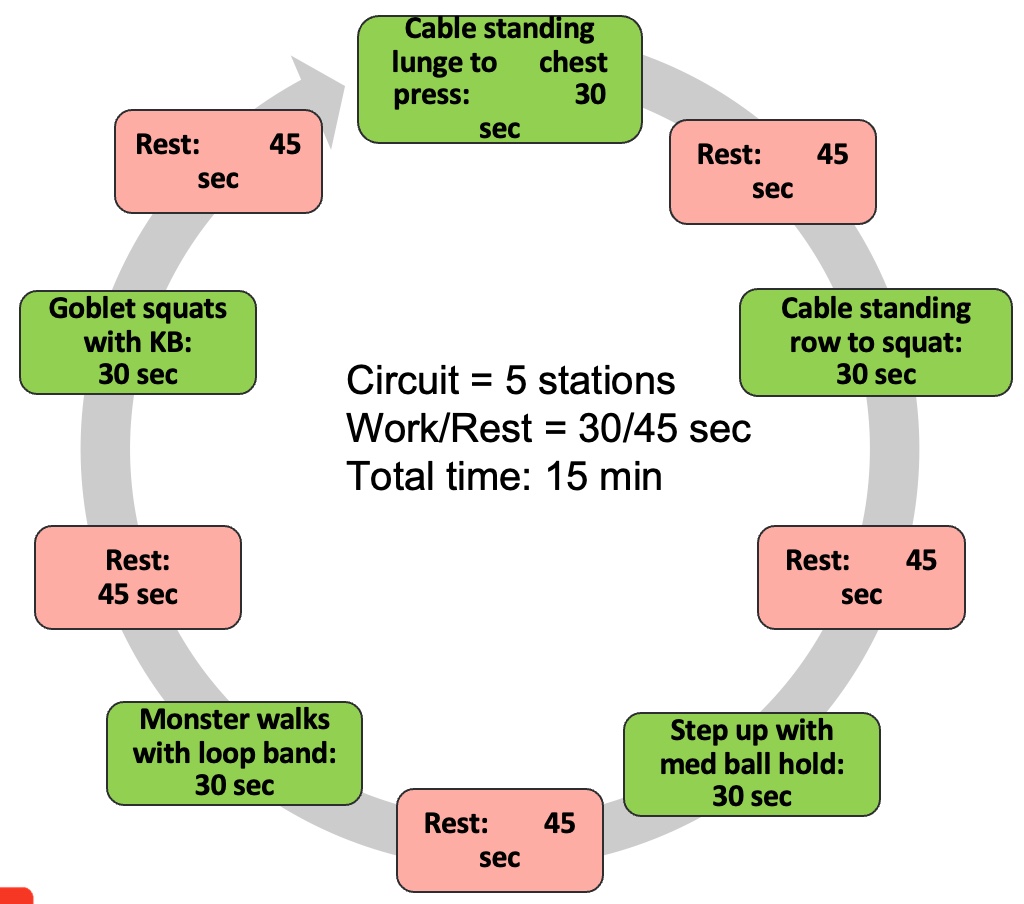
Figure 35. Resistance exercises HIIT example (beginner).
HIIT Programming
- Intensity: 65% to 100%
- Load: 40% to 65% 1RM
- Work time: 30 sec
- Rest time: 45 sec
- Total exercise time: 15 min
- Warm-up/cool-down
Intermediate participants engage in more challenging exercises such as dumbbell plank rows, farmer's carries with weights, step up to dumbbell press, kettlebell swings, and barbell Romanian deadlifts. These exercises target a higher intensity level, reflecting the participants' improved strength and capability.
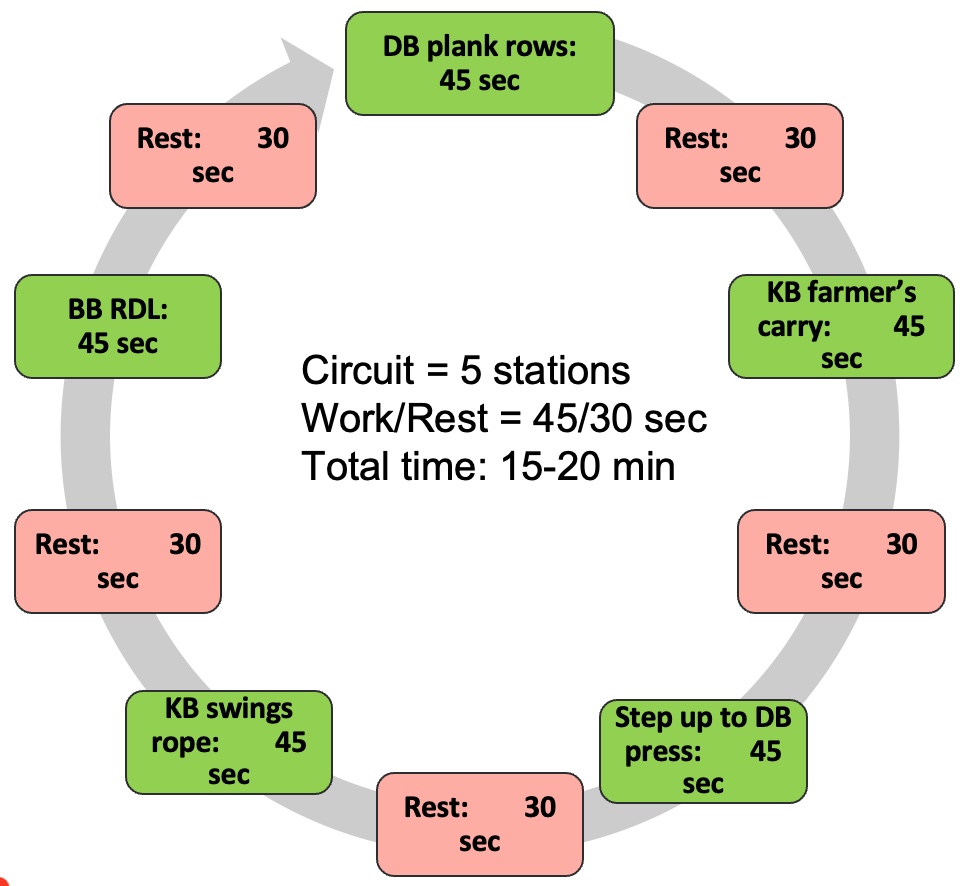
Figure 36. Resistance exercises HIIT example (intermediate).
HIIT Programming
- Intensity: 75% to 100%
- Load: 40% to 65% 1RM
- Work time: 45 sec
- Rest time: 30 sec
- Total exercise time: 15 to 20 min
- Warm-up/cool-down
Advanced resistance training involves exercises like medicine ball slams, sled push-pull, trap bar deadlifts, barbell squat with press, and medicine ball rotations. These exercises are designed for individuals with greater strength and fitness levels, enabling them to tackle more demanding workouts.
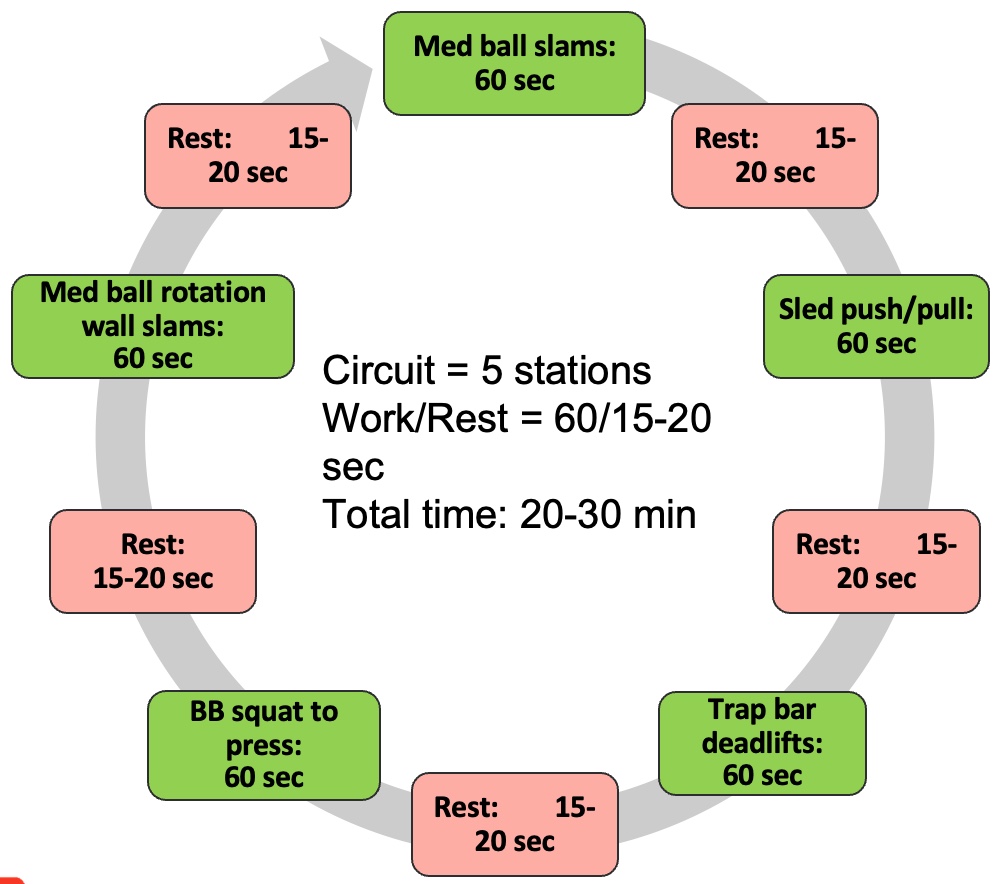
Figure 37. Resistance exercises HIIT example (advanced).
HIIT Programming
- Intensity: 85% to 100%
- Load: 40% to 65% 1RM
- Work time: 60 seconds
- Rest time: 15 to 20 seconds
- Total exercise time: 20 to 30 min
- Warm-up/cool-down
By tailoring resistance training exercises to different proficiency levels, individuals can gradually progress and challenge themselves while ensuring safety and effectiveness.
HIIT Example: Mixed (Short-Intervals)
In mixed short intervals, versatility reigns supreme, offering clients a diverse and engaging workout regimen. For beginners, maintaining an intensity level of around 65% and incorporating a load of 40% to 60% is ideal. However, it's crucial to note that the recommended load falls within the 40% to 65% range for HIIT training due to the need to sustain proper form during high-intensity efforts. A mixture of body weight and weighted exercises is recommended to mitigate injury risks. For instance, a circuit might include planks, medicine ball squats, loop band walks, jumping jacks, and kettlebell exercises.
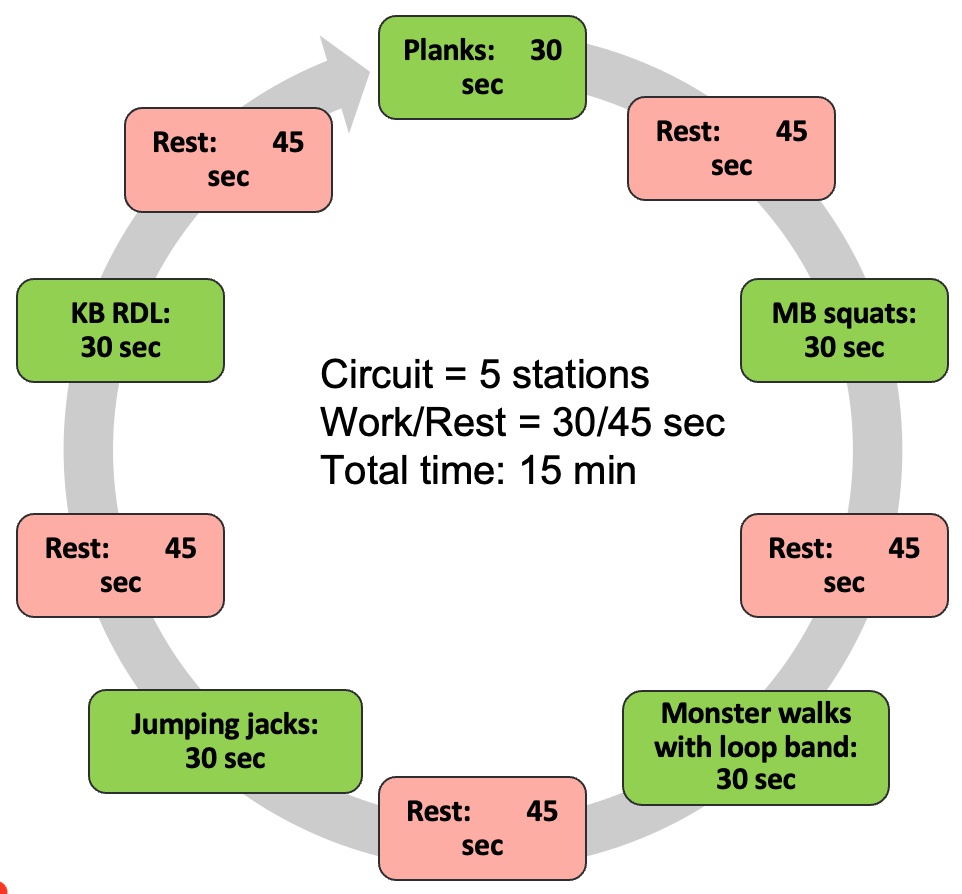
Figure 38. Mixed exercises HIIT example (beginner).
HIIT Programming
- Intensity: 65 to 100%
- Load: BW or 40% to 65% 1RM
- Work time: 30 sec
- Rest time: 45 sec
- Total exercise time: 15 min
- Warm-up/cool-down
Intermediate participants follow a similar approach, combining body weight and weighted exercises to challenge themselves while adhering to appropriate work-to-rest ratios. This ensures that they can push themselves to their limits without compromising form or safety.
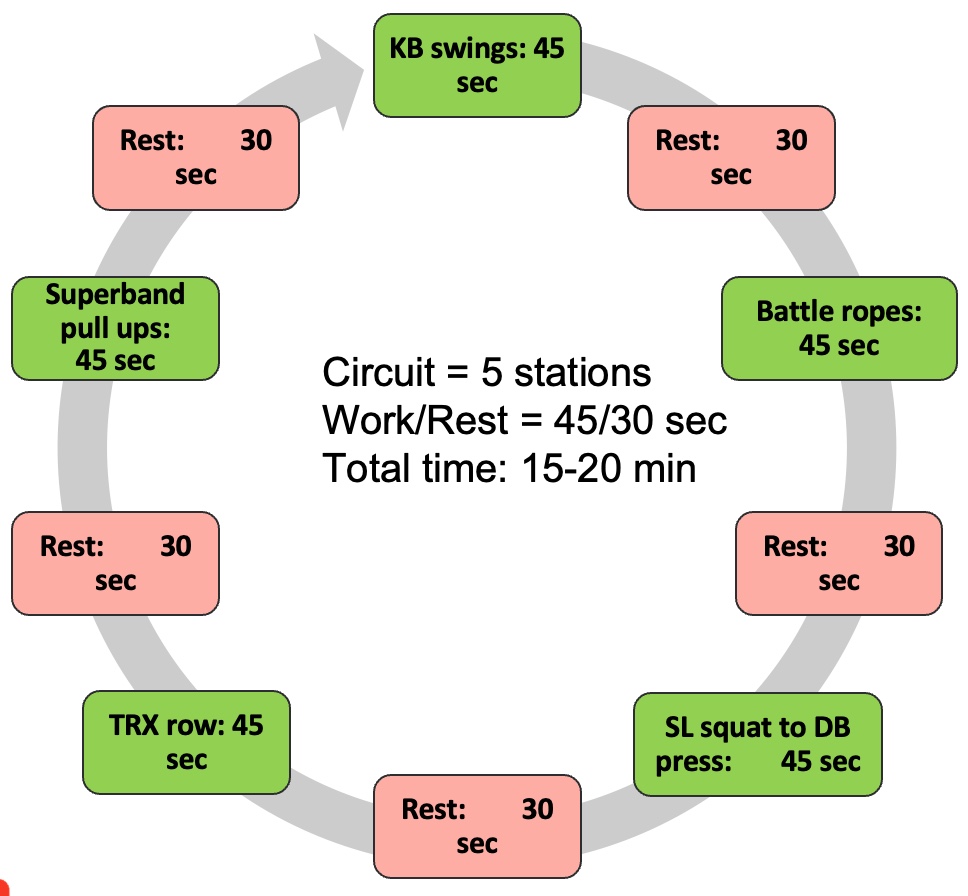
Figure 39. Mixed exercises HIIT example (intermediate).
HIIT Programming
- Intensity: 75% to 100%
- Load: BW or 40% to 65% 1RM
- Work time: 45 sec
- Rest time: 30 sec
- Total exercise time: 15 to 20 min
- Warm-up/cool-down
Advanced individuals ramp up the intensity, pushing themselves to their limits with a variety of challenging exercises. By maintaining a balance between high-intensity efforts and adequate recovery periods, they can maximize the effectiveness of their workouts while minimizing the risk of injury.
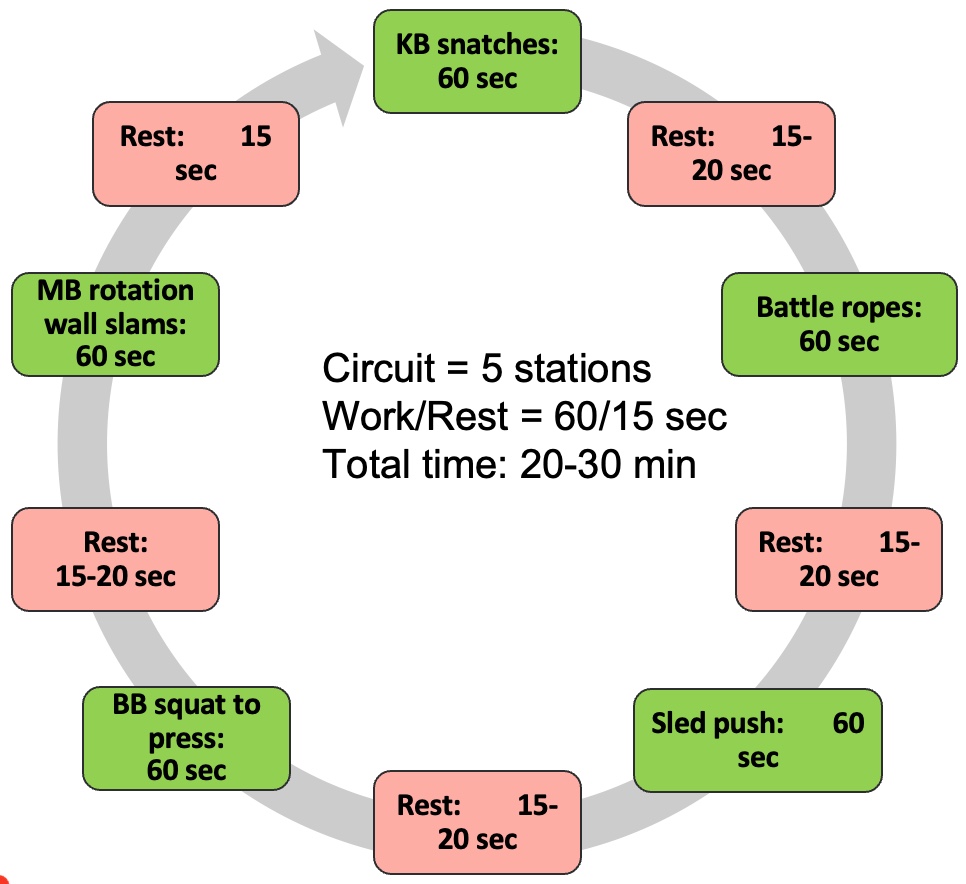
Figure 40. Mixed exercises HIIT example (advanced).
HIIT Programming
- Intensity: 75% to 100%
- Load: BW or 40% to 65% 1RM
- Work time: 60 sec
- Rest time: 15 to 20 sec
- Total exercise time: 20 to 30 min
- Warm-up/cool-down
By incorporating mixed short intervals into their workout routines, clients can enjoy a dynamic and varied training experience that targets different muscle groups and energy systems. This approach fosters both physical and mental engagement, promoting adherence and long-term success.
HIIT Example: Tabata (Short-Intervals)
Tabata training offers a compact yet intense workout option, making it ideal for clients with limited time for exercise. Developed by Dr. Tabata, this method involves alternating between 20 seconds of high-intensity exercise and 10 seconds of rest, repeated for a total of four minutes. The intensity level typically ranges from 80% to 100%, challenging participants to push their limits within a short timeframe.
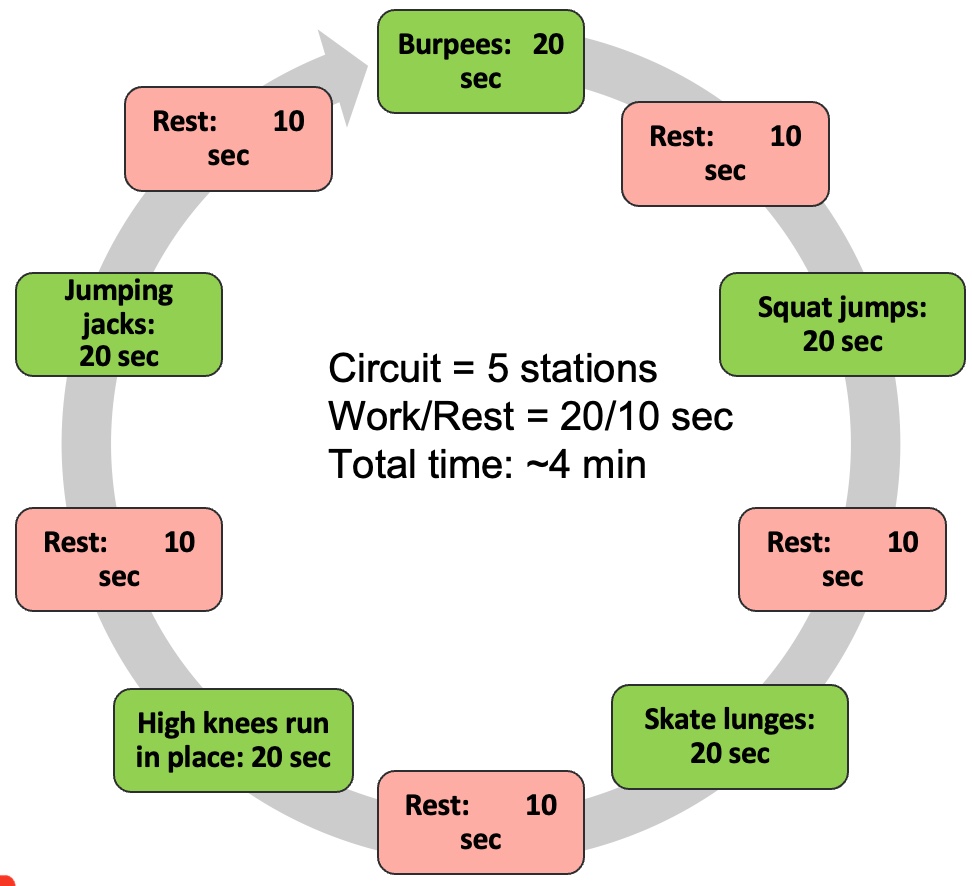
Figure 41. Tabata HIIT training, short intervals (BW advanced)
HIIT Programming
- Intensity: 85% to 100%
- Load: BW or 40% to 65% 1RM
- Work time: 20 sec
- Rest time: 10 sec
- Total exercise time: ~ 4 min
- Warm-up/cool-down
Despite its brevity, Tabata training has been shown to deliver significant benefits, making it a valuable addition to home exercise programs and busy schedules. Dr. Tabata's clinical commentary in 2019 reaffirmed the efficacy of this approach, emphasizing its potential for improving cardiovascular fitness and overall conditioning.
While Tabata training is highly effective, it's important to note that it's also advanced and may not be suitable for everyone. Clients should be adequately prepared for the intensity and duration of Tabata workouts to ensure safety and effectiveness. Nonetheless, for those who can handle the challenge, Tabata training offers a time-efficient and powerful means of improving fitness and achieving health goals.
Bottom Line
In summary, while high-intensity interval training (HIIT) offers promising benefits, it's essential to recognize that it may not be suitable for everyone. In cases where HIIT is not appropriate, alternatives such as resistance training or moderate-intensity continuous training (MICT) can be considered. It's crucial to assess each patient's suitability for HIIT and to start with lighter intensity levels before progressing. The examples provided are based on real-world application in clinical settings and can serve as a starting point for implementation.
As for the current state of research, while there is limited evidence available for resistance training and HIIT, I've presented the latest findings and guidelines to date. As research continues to evolve, we can expect further updates and refinements in the future.
Things to think about:
- Professionals should consider that HIIT training is an effective exercise to combat sarcopenia.
- HIIT may not be for every individual due to the injury risk or high intensity activity. Individuals must be monitored closely during activity.
- MICT or low load HIIT may be alternatives for some individuals with low functional capacity.
- Professionals should consider that matching the best type of HIIT to their patients may yield the best results.
Module VI: Documentation and Management
In terms of management best practices, thorough documentation is crucial. Figure 42 shows many of the items that should be addressed in your documentation.

Figure 42. Documentation best practices. (Click here to enlarge this image.)
Additionally, be aware of the new diagnostic code for sarcopenia, M62.84, especially for billing purposes. Consider integrating services for patients with sarcopenia into your practice, whether it's cash-based or otherwise. As a final reminder, prioritize resistance exercise (RE) and high-intensity interval training (HIIT) as the primary interventions. However, don't overlook the importance of comprehensive programming beyond exercise selection.
Reflect on the complexity of sarcopenia beyond its historical understanding. The assessment process is paramount, as is ongoing monitoring to gauge effectiveness and ensure patient understanding of the benefits. Lastly, consider incorporating mixed training models to enhance client engagement and enjoyment.
Final Thoughts
Things to think about:
- Sarcopenia treatment: RE/HIIT are 1st line of treatment
- Sarcopenia assessment: Clinical and fitness
- Patient monitoring: Biometric devices
- Resistance exercise & HIIT
- Integrative Training models (mixed training)
Questions and Answers
Is the DEXA scan considered the gold standard for sarcopenia?
In terms of cost-effectiveness, yes. Although CT and MRI scans are available, the DEXA scan emerges as the functional gold standard. It offers a more affordable alternative and avoids radiation exposure, making it a preferred choice. However, it's important to note that the DEXA scan has its own limitations and considerations.
Is muscle size a reliable indicator of strength?
While cross-sectional area serves as an indicator of strength, recent research highlights a discrepancy. Some individuals diagnosed with sarcopenia demonstrate strength gains without a corresponding increase in muscle size. This finding introduces a level of interpretation into the relationship between muscle size and strength.
Indeed, a nuanced argument exists regarding muscle size as a reliable indicator of strength. For instance, individuals classified as "skinny fat," characterized by low muscle mass and higher adipose tissue, challenge traditional assumptions. Hence, while muscle size remains a factor, its direct correlation with strength may not always hold true.
Can you use a sarcopenia CBT code, even if you're not on a diagnosis from the doctor, but it was obvious the initial evaluation has it?
Absolutely. I've consistently utilized the sarcopenia CBT code, both for in-network and out-of-network services, and it's been accepted without issue. Even if a formal diagnosis hasn't been provided by a physician, if the initial evaluation clearly indicates sarcopenia, billing under the corresponding ICD-10 code is appropriate.
Moreover, if your practice offers fitness and health services, integrating this code into your billing practices is worthwhile. As awareness of sarcopenia grows and it becomes more widely recognized, its acceptance in billing procedures is likely to increase.
Why are we doing HIIT if aerobic isn't really helpful for sarcopenia?
When considering the effectiveness of HIIT for sarcopenia, it's important to recognize its unique benefits. HIIT serves as a method of loading the body. It encompasses various exercises, whether utilizing weighted objects or solely relying on body weight, making it a versatile approach. By incorporating resistance and leveraging gravity, HIIT engages both the aerobic and anaerobic systems, thus presenting a hybrid form of training. HIIT offers a comprehensive workout that effectively stresses the entire body, whether with a medicine ball or traditional resistance training objects.
References
Ackermans, L. L. G. C., Rabou, J., Basrai, M., et al. (2022). Screening, diagnosis and monitoring of sarcopenia: When to use which tool? Clinical Nutrition ESPEN, 48, 36-44. https://doi.org/10.1016/j.clnesp.2022.01.027
Ardeljan, A. D., & Hurezeanu, R. (2023). Sarcopenia. Statpearls. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.
Atakan, M. M., Li, Y., Koşar, Ş. N., Turnagöl, H. H., & Yan, X. (2021). Evidence-based effects of high-intensity interval training on exercise capacity and health: A review with historical perspective. International Journal of Environmental Research and Public Health, 18(13). https://doi.org/10.3390/ijerph18137201
Baker, B. S., Stannard, M. S., Duren, D. L., Cook, J. L., & Stannard, J. P. (2020). Does blood flow restriction therapy in patients older than age 50 result in muscle hypertrophy, increased strength, or greater physical function? A systematic review. Clinical Orthopaedics and Related Research, 478(3), 593-606. https://doi.org/10.1097/corr.0000000000001090
Bhasin, S., Travison, T. G., Manini, T. M., et al. (2020). Sarcopenia definition: The position statements of the sarcopenia definition and outcomes consortium. Journal of the American Geriatrics Society, 68(7), 1410-1418. https://doi.org/10.1111/jgs.16372
Blanquet, M., Ducher, G., Sauvage, A., et al. (2022). Handgrip strength as a valid practical tool to screen early-onset sarcopenia in acute care wards: A first evaluation. European Journal of Clinical Nutrition, 76(1), 56-64. https://doi.org/10.1038/s41430-021-00906-5
Buchheit, M., & Laursen, P. B. (2013). High-intensity interval training, solutions to the programming puzzle: Part i: Cardiopulmonary emphasis. Sports Medicine, 43(5), 313-338. https://doi.org/10.1007/s40279-013-0029-x
Cannataro, R., Carbone, L., Petro, J. L., et al. (2021). Sarcopenia: Etiology, nutritional approaches, and mirnas. International Journal of Molecular Sciences, 22(18). https://doi.org/10.3390/ijms22189724
Cesari, M., Kritchevsky, S. B., Newman, A. B., et al. (2009). Added value of physical performance measures in predicting adverse health-related events: Results from the health, aging and body composition study. Journal of the American Geriatrics Society, 57(2), 251-259. https://doi.org/10.1111/j.1532-5415.2008.02126.x
Cheatham, S. W., & Cain, M. (2015). Rheumatoid arthritis: Exercise programming for the strength and conditioning professional. Strength & Conditioning Journal, 37(1), 30-39. https://doi.org/10.1519/ssc.0000000000000117
Chen, L. K., Woo, J., Assantachai, P., et al. (2020). Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. Journal of the American Medical Directors Association, 21(3), 300-307.e2. https://doi.org/10.1016/j.jamda.2019.12.012
Chen, N., He, X., Feng, Y., Ainsworth, B. E., & Liu, Y. (2021). Effects of resistance training in healthy older people with sarcopenia: A systematic review and meta-analysis of randomized controlled trials. European Review of Aging and Physical Activity, 18(1), 23. https://doi.org/10.1186/s11556-021-00277-7
Chen, X., Hou, L., Zhang, Y., Luo, S., & Dong, B. (2021). The accuracy of the ishii score chart in predicting sarcopenia in the elderly community in Chengdu. BMC Geriatrics, 21(1), 296. https://doi.org/10.1186/s12877-021-02244-4
Cruz-Jentoft, A. J., Baeyens, J. P., Bauer, J. M., et al. (2010). Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age and Ageing, 39(4), 412-423. https://doi.org/10.1093/ageing/afq034
Cruz-Jentoft, A. J., Bahat, G., Bauer, J., et al. (2018). Sarcopenia: Revised European consensus on definition and diagnosis. Age and Ageing, 48(1), 16-31. https://doi.org/10.1093/ageing/afy169
De Spiegeleer, A., Beckwée, D., Bautmans, I., & Petrovic, M. (2018). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Drugs & Aging, 35(8), 719-734. https://doi.org/10.1007/s40266-018-0566-y
Dodds, R. M., Syddall, H. E., Cooper, R., et al. (2014). Grip strength across the life course: Normative data from twelve British studies. PLoS One, 9(12), e113637. https://doi.org/10.1371/journal.pone.0113637
Erdogan, T., Catikkas, N. M., Oren, M. M., et al. (2022). Ishii test for screening sarcopenia: Performance in community-dwelling older adults. Aging Clinical and Experimental Research, 34(4), 785-791. https://doi.org/10.1007/s40520-021-01998-6
Fried, L. P., Tangen, C. M., Walston, J., et al. (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology: Series A, 56(3), M146-M156. https://doi.org/10.1093/gerona/56.3.m146
Geng, Q., Zhai, H., Wang, L., Wei, H., & Hou, S. (2023). The efficacy of different interventions in the treatment of sarcopenia in middle-aged and elderly people: A network meta-analysis. Medicine, 102(27), e34254. https://doi.org/10.1097/md.0000000000034254
Gielen, E., Beckwée, D., Delaere, A., et al. (2021). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutrition Reviews, 79(2), 121-147. https://doi.org/10.1093/nutrit/nuaa011
Grimby, G., & Saltin, B. (1983). The ageing muscle. Clinical Physiology, 3(3), 209-218. https://doi.org/10.1111/j.1475-097x.1983.tb00704.x
Guttikonda, D., & Smith, A. L. (2021). Sarcopenia assessment techniques. Clinical Liver Disease (Hoboken), 18(4), 189-192. https://doi.org/10.1002/cld.1111
Hayes, L. D., Elliott, B. T., Yasar, Z., et al. (2021). High intensity interval training (HIIT) as a potential countermeasure for phenotypic characteristics of sarcopenia: A scoping review. Frontiers in Physiology, 12, 715044. https://doi.org/10.3389/fphys.2021.715044
Helms, E. R., Storey, A., Cross, M. R., et al. (2017). RPE and velocity relationships for the back squat, bench press, and deadlift in powerlifters. Journal of Strength and Conditioning Research, 31(2), 292-297. https://doi.org/10.1519/jsc.0000000000001517
Herrod, P. J. J., Atherton, P. J., Smith, K., et al. (2021). Six weeks of high-intensity interval training enhances contractile activity induced vascular reactivity and skeletal muscle perfusion in older adults. Geroscience, 43(6), 2667-2678. https://doi.org/10.1007/s11357-021-00463-6
Hurst, C., Robinson, S. M., Witham, M. D., et al. (2022). Resistance exercise as a treatment for sarcopenia: Prescription and delivery. Age and Ageing. https://doi.org/10.1093/ageing/afac003
Ida, S., Kaneko, R., & Murata, K. (2018). SARC-F for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. Journal of the American Medical Directors Association, 19(8), 685-689. https://doi.org/10.1016/j.jamda.2018.04.001
Ishii, S., Tanaka, T., Shibasaki, K., et al. (2014). Development of a simple screening test for sarcopenia in older adults. Geriatrics & Gerontology International, 14(S1), 93-101. https://doi.org/10.1111/ggi.12197
Kim, H. I., & Kim, M. C. (2021). Physical therapy assessment tool threshold values to identify sarcopenia and locomotive syndrome in the elderly. International Journal of Environmental Research and Public Health, 20(12). https://doi.org/10.3390/ijerph20126098
Kim, J. W., Kim, R., Choi, H., Lee, S.-J., & Bae, G.-U. (2021). Understanding of sarcopenia: From definition to therapeutic strategies. Archives of Pharmacal Research, 44(9), 876-889. https://doi.org/10.1007/s12272-021-01349-z
Krzymińska-Siemaszko, R., Deskur-Śmielecka, E., Kaluźniak-Szymanowska, A., Lewandowicz, M., & Wieczorowska-Tobis, K. (2020). Comparison of diagnostic performance of SARC-F and its two modified versions (SARC-CALF and SARC-F+ebm) in community-dwelling older adults from Poland. Clinical Interventions in Aging, 15, 583-594. https://doi.org/10.2147/cia.S250508
Kumar, P., Umakanth, S., & Girish, N. (2022). A review of the components of exercise prescription for sarcopenic older adults. European Geriatric Medicine, 13(6), 1245-1280. https://doi.org/10.1007/s41999-022-00693-7
Law, T. D., Clark, L. A., & Clark, B. C. (2016). Resistance exercise to prevent and manage sarcopenia and dynapenia. Annual Review of Gerontology and Geriatrics, 36(1), 205-228. https://doi.org/10.1891/0198-8794.36.205
Lea, J. W. D., O'Driscoll, J. M., Hulbert, S., Scales, J., & Wiles, J. D. (2022). Convergent validity of ratings of perceived exertion during resistance exercise in healthy participants: A systematic review and meta-analysis. Sports Medicine - Open, 8(1), 2. https://doi.org/10.1186/s40798-021-00386-8
Lee, S. H., & Gong, H. S. (2020). Measurement and interpretation of handgrip strength for research on sarcopenia and osteoporosis. Journal of Bone Metabolism, 27(2), 85-96. https://doi.org/10.11005/jbm.2020.27.2.85
Lian, R., Jiang, G., Liu, Q., et al. (2023). Validated tools for screening sarcopenia: A scoping review. Journal of the American Medical Directors Association. https://doi.org/10.1016/j.jamda.2023.06.036
Lin, Y.-L., Wang, C.-H., Tsai, J.-P., et al. (2022). A comparison of SARC-F, calf circumference, and their combination for sarcopenia screening among patients undergoing peritoneal dialysis. Nutrients, 14(5), 923. https://doi.org/10.3390/nu14050923
Liu, Q. Q., Xie, W. Q., Luo, Y. X., et al. (2022). High intensity interval training: A potential method for treating sarcopenia. Clinical Interventions in Aging, 17, 857-872. https://doi.org/10.2147/cia.S366245
Long, Y. F., Chow, S. K., Cui, C., et al. (2023). Does exercise influence skeletal muscle by modulating mitochondrial functions via regulating micrornas? A systematic review. Ageing Research Reviews, 91, 102048. https://doi.org/10.1016/j.arr.2023.102048
Lu, L., Mao, L., Feng, Y., et al. (2021). Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: A systematic review and meta-analysis. BMC Geriatrics, 21(1), 708. https://doi.org/10.1186/s12877-021-02642-8
Luo, S., Chen, X., Hou, L., et al. (2021). Cut-off points of the ishii test to diagnosing severe sarcopenia among multi-ethnic middle-aged to older adults: Results from the West China health and aging trend study. Frontiers in Medicine, 10. https://doi.org/10.3389/fmed.2023.1176128
Marcangeli, V., Youssef, L., Dulac, M., et al. (2022). Impact of high-intensity interval training with or without l-citrulline on physical performance, skeletal muscle, and adipose tissue in obese older adults. Journal of Cachexia, Sarcopenia and Muscle, 13(3), 1526-1540. https://doi.org/10.1002/jcsm.12955
Martin-Smith, R., Cox, A., Buchan, D. S., et al. (2020). High intensity interval training (HIIT) improves cardiorespiratory fitness (CRF) in healthy, overweight and obese adolescents: A systematic review and meta-analysis of controlled studies. International Journal of Environmental Research and Public Health, 17(8). https://doi.org/10.3390/ijerph17082955
Miljkovic, N., Lim, J. Y., Miljkovic, I., & Frontera, W. R. (2015). Aging of skeletal muscle fibers. Annals of Rehabilitation Medicine, 39(2), 155-162. https://doi.org/10.5535/arm.2015.39.2.155
Miller, D. K., Malmstrom, T. K., Andresen, E. M., et al. (2009). Development and validation of a short portable sarcopenia measure in the African American health project. Journal of Gerontology: Medical Sciences, 64(3), 388-394. https://doi.org/10.1093/gerona/gln033
Newman, A. B., Simonsick, E. M., Naydeck, B. L., et al. (2006). Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA, 295(17), 2018-2026. https://doi.org/10.1001/jama.295.17.2018
Nilwik, R., Snijders, T., Leenders, M., et al. (2013). The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Experimental Gerontology, 48(5), 492-498. https://doi.org/10.1016/j.exger.2013.02.012
Nishikawa, H., Asai, A., Fukunishi, S., et al. (2021). Screening tools for sarcopenia. In Vivo, 35(6), 3001-3009. https://doi.org/10.21873/invivo.12595
Offord, N. J., & Witham, M. D. (2017). The emergence of sarcopenia as an important entity in older people. Clinical Medicine (London, England), 17(4), 363-366. https://doi.org/10.7861/clinmedicine.17-4-363
Pavasini, R., Guralnik, J., Brown, J. C., et al. (2016). Short physical performance battery and all-cause mortality: Systematic review and meta-analysis. BMC Medicine, 14(1), 215. https://doi.org/10.1186/s12916-016-0763-7
Roberts, H. C., Denison, H. J., Martin, H. J., et al. (2011). A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age and Ageing, 40(4), 423-429. https://doi.org/10.1093/ageing/afr051
Sawada, S., Ozaki, H., Natsume, T., et al. (2021). The 30-s chair stand test can be a useful tool for screening sarcopenia in elderly Japanese participants. BMC Musculoskeletal Disorders, 22(1), 639. https://doi.org/10.1186/s12891-021-04524-x
Sayer, A. A., & Cruz-Jentoft, A. (2022). Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age and Ageing, 51(10). https://doi.org/10.1093/ageing/afac220
Shafiee, G., Keshtkar, A., Soltani, A., et al. (2017). Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. Journal of Diabetes & Metabolic Disorders, 16(1), 21. https://doi.org/10.1186/s40200-017-0302-x
Shen, Y., Shi, Q., Nong, K., et al. (2023). Exercise for sarcopenia in older people: A systematic review and network meta-analysis. Journal of Cachexia, Sarcopenia and Muscle, 14(3), 1199-1211. https://doi.org/10.1002/jcsm.13225
Stuck, A. K., Tsai, L. T., Freystaetter, G., et al. (2023). Comparing prevalence of sarcopenia using twelve sarcopenia definitions in a large multinational European population of community-dwelling older adults. The Journal of Nutrition, Health & Aging, 27(3), 205-212. https://doi.org/10.1007/s12603-023-1888-y
Su, H., Wen, T., Liu, D., et al. (2022). Effect of 32-weeks high-intensity interval training and resistance training on delaying sarcopenia: Focus on endogenous apoptosis. Frontiers in Physiology, 13, 811369. https://doi.org/10.3389/fphys.2022.811369
Tabata, I. (2019). Tabata training: One of the most energetically effective high-intensity intermittent training methods. The Journal of Physiological Sciences, 69(4), 559-572. https://doi.org/10.1007/s12576-019-00676-7
Tang, T., Wu, L., Yang, L., et al. (2018). A sarcopenia screening test predicts mortality in hospitalized older adults. Scientific Reports, 8(1), 2923. https://doi.org/10.1038/s41598-018-21237-9
Vlietstra, L., Waters, D. L., Jones, L. M., et al. (2023). High-intensity interval aerobic and resistance training to counteract low relative lean soft tissue mass in middle age: A randomized controlled trial. Experimental Gerontology, 171, 111991. https://doi.org/10.1016/j.exger.2020.111991
Voelker, S. N., Michalopoulos, N., Maier, A. B., & Reijnierse, E. M. (2021). Reliability and concurrent validity of the SARC-F and its modified versions: A systematic review and meta-analysis. Journal of the American Medical Directors Association, 22(9), 1864-1876.e16. https://doi.org/10.1016/j.jamda.2021.05.011
Vollaard, N. B. J., & Metcalfe, R. S. (2017). Research into the health benefits of sprint interval training should focus on protocols with fewer and shorter sprints. Sports Medicine, 47(12), 2443-2451. https://doi.org/10.1007/s40279-017-0727-x
Volpi, E., Nazemi, R., & Fujita, S. (2004). Muscle tissue changes with aging. Current Opinion in Clinical Nutrition and Metabolic Care, 7(4), 405-410. https://doi.org/10.1097/01.mco.0000134362.76653.b2
von Haehling, S., Morley, J. E., & Anker, S. D. (2010). An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. Journal of Cachexia, Sarcopenia and Muscle, 1(2), 129-133. https://doi.org/10.1007/s13539-010-0014-2
Wang, J., Jiang, Y., Wang, Y., et al. (2022). Association between sarcopenia and metabolic syndrome in middle-aged and older non-obese adults: A systematic review and meta-analysis. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 15, 1491-1502. https://doi.org/10.2147/dmso.s367584
Wu, Z. J., Wang, Z. Y., Gao, H. E., Zhou, X. F., & Li, F. H. (2021). Impact of high-intensity interval training on cardiorespiratory fitness, body composition, physical fitness, and metabolic parameters in older adults: A meta-analysis of randomized controlled trials. Experimental Gerontology, 150, 111345. https://doi.org/10.1016/j.exger.2021.111345
Xu, B., Guo, Z., Jiang, B., et al. (2022). Factors affecting sarcopenia in older patients with chronic diseases. Annals of Palliative Medicine, 11(3), 972-983.
Xue, Q. L. (2011). The frailty syndrome: Definition and natural history. Clinics in Geriatric Medicine, 27(1), 1-15. https://doi.org/10.1016/j.cger.2010.08.009
Yamada, M., Kimura, Y., Ishiyama, D., et al. (2018). Differential characteristics of skeletal muscle in community-dwelling older adults. Journal of the American Medical Directors Association, 19(4), 311-316. https://doi.org/10.1016/j.jamda.2017.10.015
Yoo, J. I., Choi, H., & Ha, Y. C. (2017). Mean hand grip strength and cut-off value for sarcopenia in Korean adults using KNHANES VI. Journal of Korean Medical Science, 32(5), 868-872. https://doi.org/10.3346/jkms.2017.32.5.868
Yoo, S. Z., No, M. H., Heo, J. W., et al. (2022). Role of exercise in age-related sarcopenia. Journal of Exercise Rehabilitation, 18(5), 367-374. https://doi.org/10.12965/jer.2142394.471
Yu, S. C., Khow, K. S., Jadczak, A. D., & Visvanathan, R. (2016). Clinical screening tools for sarcopenia and its management. Current Gerontology and Geriatrics Research, 2016, 5978523. https://doi.org/10.1155/2016/5978523
Yuan, S., & Larsson, S. C. (2023). Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism - Clinical and Experimental, 144. https://doi.org/10.1016/j.metabol.2023.155533
Zhang, H., Lin, S., Gao, T., et al. (2022). Comparison of the diagnostic performance of the calf circumference, gait speed and handgrip strength in older patients with sarcopenia. Aging Clinical and Experimental Research. https://doi.org/10.1007/s40520-022-02126-x
Zhang, Y., Li, X., Liu, L., et al. (2018). Therapeutic effect of high-intensity interval training on sarcopenia in elderly patients with chronic heart failure. Cellular Physiology and Biochemistry, 45(4), 1435-1446. https://doi.org/10.1159/000487582
Zeng, D., Ling, X.-Y., Fang, Z.-L., & Lu, Y.-F. (2023). Optimal exercise to improve physical ability and performance in older adults with sarcopenia: A systematic review and network meta-analysis. Geriatric Nursing, 52, 199-207. https://doi.org/10.1016/j.gerinurse.2023.06.005
Zhou, Z., Zhou, R., Zhu, Y., et al. (2021). Association between sarcopenia and health-related quality of life among older adults: A systematic review and meta-analysis. Journal of the American Medical Directors Association. https://doi.org/10.1016/j.jamda.2023.06.036
Zhu, Q., Shen, X., & Qin, Y. (2022). Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: A systematic review and meta-analysis. The Journal of Nutrition, Health & Aging, 26(10), 1355-1364. https://doi.org/10.1007/s12603-022-2080-9
Zhu, Y., Zhou, X., Zhu, A., et al. (2023). Advances in exercise to alleviate sarcopenia in older adults by improving mitochondrial dysfunction. Frontiers in Physiology, 14, 1196426. https://doi.org/10.3389/fphys.2023.1196426
Zhuang, C.-L., Shen, X., Zou, H.-B., et al. (2019). High-intensity interval training improves functional capacity and quality of life in patients with post-acute myocardial infarction: A randomized controlled trial. BMC Cardiovascular Disorders, 19(1), 223. https://doi.org/10.1186/s12872-019-1201-3
Citation
Cheatham, S. (2023). Resistance training and HIIT: Implications for sarcopenia and longevity. PhysicalTherapy.com, Article 4904. Available at www.PhysicalTherapy.com
